Impact of body mass index on real-world outcomes of rivaroxaban treatment in Japanese patients with non-valvular atrial fibrillation
- PMID: 32253531
- PMCID: PMC7332477
- DOI: 10.1007/s00380-020-01587-z
Impact of body mass index on real-world outcomes of rivaroxaban treatment in Japanese patients with non-valvular atrial fibrillation
Abstract
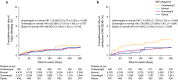
This sub-analysis of the XAPASS, a prospective, single-arm, observational study, aimed to evaluate relationships between body mass index (BMI) and safety (major bleeding and all-cause mortality) and effectiveness [stroke/non-central nervous system (non-CNS) systemic embolism (SE)/myocardial infarction (MI)] outcomes in Japanese patients with non-valvular atrial fibrillation (NVAF) receiving rivaroxaban. Patients were categorized according to BMI (kg/m2) as underweight (< 18.5), normal weight (18.5 to < 25), overweight (25 to < 30), or obese (≥ 30). In total, 9578 patients with NVAF completed the 1-year follow-up and were evaluated; of these, 7618 patients had baseline BMI data. Overall, 542 (5.7%), 4410 (46.0%), 2167 (22.6%), and 499 (5.2%) patients were underweight, normal weight, overweight, and obese, respectively. Multivariable Cox regression analysis demonstrated that none of the BMI categories were independent predictors of major bleeding whereas being underweight was independently associated with increased all-cause mortality [hazard ratio (HR) 3.56, 95% confidence interval (CI) 2.40-5.26, p < 0.001]. The incidence of stroke/non-CNS SE/MI was higher in patients who were underweight than in those of normal weight (HR 2.11, 95% CI 1.20-3.70, p = 0.009). However, in multivariable analyses, being underweight was not identified as an independent predictor of stroke/non-CNS SE/MI (HR 1.64, 95% CI 0.90-2.99, p = 0.104). In conclusion, the high incidence of thromboembolic events and all-cause mortality in patients who were underweight highlights that thorough evaluation of disease status and comorbidities may be required in this population.
Keywords: Atrial fibrillation; Body mass index; Rivaroxaban; Stroke.
Conflict of interest statement
YM, TI, SO, TK, JN, KM, and SM were advisory board members for Bayer Yakuhin, Ltd. YM received research grants from Bayer Yakuhin, Ltd., Boehringer Ingelheim, and Daiichi Sankyo, and honoraria from Bayer Yakuhin, Ltd., Boehringer Ingelheim, Bristol-Myers Squibb, and Daiichi Sankyo. TI received research grants from Bayer Yakuhin, Ltd., Bristol-Myers Squibb, Daiichi Sankyo, Medtronic Japan, and St. Jude Medical, and honoraria from Bayer Yakuhin, Ltd., Bristol-Myers Squibb, Daiichi Sankyo, Ono, and Pfizer; TI was an advisory board member for Bristol-Myers Squibb. TK received a research grant from Bayer Yakuhin, Ltd. JN received a research grant from Nihon Medi-Physics. KM received honoraria from Astellas, AstraZeneca, Bayer Yakuhin, Ltd., BMS, Boehringer Ingelheim, Daiichi Sankyo, Japan Stryker, Kowa, Mitsubishi-Tanabe, Nihon Medi-Physics, Nippon Chemiphar, Otsuka, Pfizer, Sawai, and Sumitomo Dainippon; KM was an advisory board member for CSL Behring and Medico’s Hirata. SM received research grants from Astellas, Brainlab, Bristol-Myers Squibb, Carl Zeiss Meditec, Chugai, CSL Behring, Daiichi Sankyo, Eisai, Medtronic, Meiji, Mitsubishi-Tanabe, MSD, Mizuho, Nihon Medi-Physics, Otsuka, Pfizer, Philips Electronics Japan, Sanofi, Siemens Healthcare, and Takeda. YH, YK, YO, TS, SS, and SY are employees of Bayer Yakuhin, Ltd.
Figures
Similar articles
-
Clinical Risk Factors of Thromboembolic and Major Bleeding Events for Patients with Atrial Fibrillation Treated with Rivaroxaban in Japan.J Stroke Cerebrovasc Dis. 2020 Apr;29(4):104584. doi: 10.1016/j.jstrokecerebrovasdis.2019.104584. Epub 2020 Jan 23. J Stroke Cerebrovasc Dis. 2020. PMID: 31983518
-
Real-world outcomes of rivaroxaban treatment in elderly Japanese patients with nonvalvular atrial fibrillation.Heart Vessels. 2020 Mar;35(3):399-408. doi: 10.1007/s00380-019-01487-x. Epub 2019 Sep 6. Heart Vessels. 2020. PMID: 31492970 Free PMC article.
-
Real-world outcomes of rivaroxaban treatment in patients with nonvalvular atrial fibrillation and worsening renal function.J Cardiol. 2019 Dec;74(6):501-506. doi: 10.1016/j.jjcc.2019.06.003. Epub 2019 Jul 29. J Cardiol. 2019. PMID: 31371191
-
Direct comparative effectiveness and safety between non-vitamin K antagonist oral anticoagulants for stroke prevention in nonvalvular atrial fibrillation: a systematic review and meta-analysis of observational studies.Eur J Epidemiol. 2019 Feb;34(2):173-190. doi: 10.1007/s10654-018-0415-7. Epub 2018 Jun 8. Eur J Epidemiol. 2019. PMID: 29948370
-
[Rivaroxaban at Non-Valvular Atrial Fibrillation: a Prospective Study and Clinical Practice].Kardiologiia. 2016 Aug;56(8):87-92. doi: 10.18565/cardio.2016.8.87-92. Kardiologiia. 2016. PMID: 28290887 Review. Russian.
Cited by
-
Safety and efficacy of direct oral anticoagulants in comparison with warfarin across different BMI ranges: A systematic review and meta-analysis.Ann Med Surg (Lond). 2022 Apr 14;77:103610. doi: 10.1016/j.amsu.2022.103610. eCollection 2022 May. Ann Med Surg (Lond). 2022. PMID: 35637978 Free PMC article. Review.
-
JCS/JHRS 2024 Guideline Focused Update on Management of Cardiac Arrhythmias.J Arrhythm. 2025 Jun 16;41(3):e70033. doi: 10.1002/joa3.70033. eCollection 2025 Jun. J Arrhythm. 2025. PMID: 40524851 Free PMC article. No abstract available.
-
Association between body mass index and long-term clinical outcomes in patients with non-valvular atrial fibrillation taking oral anticoagulants.Heart Vessels. 2023 Apr;38(4):551-561. doi: 10.1007/s00380-022-02194-w. Epub 2022 Nov 4. Heart Vessels. 2023. PMID: 36331618
-
The impact of underweight and obesity on outcomes in anticoagulated patients with atrial fibrillation: A systematic review and meta-analysis on the obesity paradox.Clin Cardiol. 2021 May;44(5):599-608. doi: 10.1002/clc.23593. Epub 2021 Mar 26. Clin Cardiol. 2021. PMID: 33769583 Free PMC article.
-
Body Mass Index Influence on the Clinical Outcomes for Nonvalvular Atrial Fibrillation Patients Admitted to a Hospital Treated with Direct Oral Anticoagulants: A Retrospective Cohort Study.Drug Des Devel Ther. 2021 May 6;15:1931-1943. doi: 10.2147/DDDT.S303219. eCollection 2021. Drug Des Devel Ther. 2021. PMID: 33986592 Free PMC article.
References
-
- Haas S, Camm AJ, Bassand JP, Angchaisuksiri P, Cools F, Corbalan R, Gibbs H, Jacobson B, Koretsune Y, Mantovani LG, Misselwitz F, Panchenko E, Ragy HI, Stepinska J, Turpie AG, Sawhney JP, Steffel J, Lim TW, Pieper KS, Virdone S, Verheugt FW, Kakkar AK. Predictors of NOAC versus VKA use for stroke prevention in patients with newly diagnosed atrial fibrillation: results from GARFIELD-AF. Am Heart J. 2019;213:35–46. doi: 10.1016/j.ahj.2019.03.013. - DOI - PubMed
-
- Miyasaka Y, Barnes ME, Gersh BJ, Cha SS, Bailey KR, Abhayaratna WP, Seward JB, Tsang TS. Secular trends in incidence of atrial fibrillation in Olmsted County, Minnesota, 1980 to 2000, and implications on the projections for future prevalence. Circulation. 2006;114(2):119–125. doi: 10.1161/CIRCULATIONAHA.105.595140. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

