cGAS/STING Pathway Activation Contributes to Delayed Neurodegeneration in Neonatal Hypoxia-Ischemia Rat Model: Possible Involvement of LINE-1
- PMID: 32253733
- PMCID: PMC7260114
- DOI: 10.1007/s12035-020-01904-7
cGAS/STING Pathway Activation Contributes to Delayed Neurodegeneration in Neonatal Hypoxia-Ischemia Rat Model: Possible Involvement of LINE-1
Abstract
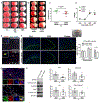
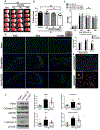
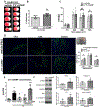
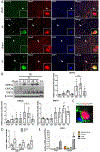
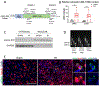
cGAS is a sensor of cytosolic DNA and responds equally to exogenous and endogenous DNA. After recognition of cytosolic dsDNA or ssDNA, cGAS synthesizes the second messenger 2'3'-cGAMP, which then binds to and activates stimulator of interferon genes (STING). STING plays an essential role in responding to pathogenic DNA and self-DNA in the context of autoimmunity. In pathologic conditions, such as stroke or hypoxia-ischemia (HI), DNA can gain access into the cytoplasm of the cell and leak from the dying cells into the extracellular environment, which potentially activates cGAS/STING. Recent in vivo studies of myocardial ischemia, traumatic brain injury, and liver damage models suggest that activation of cGAS/STING is not only a side-effect of the injury, but it can also actively contribute to cell death and apoptosis. We found, for the first time, that cGAS/STING pathway becomes activated between 24 and 48 h after HI in a 10-day-old rat model. Silencing STING with siRNA resulted in decreased infarction area, reduced cortical neurodegeneration, and improved neurobehavior at 48 h, suggesting that STING can contribute to injury progression after HI. STING colocalized with lysosomal marker LAMP-1 and blocking STING reduced the expression of cathepsin B and decreased the expression of Bax and caspase 3 cleavage. We observed similar protective effects after intranasal treatment with cGAS inhibitor RU.521, which were reversed by administration of STING agonist 2'3'-cGAMP. Additionally, we showed that long interspersed element 1 (LINE-1) retrotransposon, a potential upstream activator of cGAS/STING pathway was induced at 48 h after HI, which was evidenced by increased expression of ORF1p and ORF2p proteins and increased LINE-1 DNA content in the cytosol. Blocking LINE-1 with the nucleoside analog reverse-transcriptase inhibitor (NRTI) stavudine reduced infarction area, neuronal degeneration in the cerebral cortex, and reduced the expression of Bax and cleaved caspase 3. Thus, our results identify the cGAS/STING pathway as a potential therapeutic target to inhibit delayed neuronal death after HI.
Keywords: Apoptosis; LINE-1; Neurodegeneration; STING; cGAS.
Conflict of interest statement
Declaration of Interest
The authors declare no conflict of interest for this study.
Figures




Similar articles
-
Activating cGAS-STING axis contributes to neuroinflammation in CVST mouse model and induces inflammasome activation and microglia pyroptosis.J Neuroinflammation. 2022 Jun 10;19(1):137. doi: 10.1186/s12974-022-02511-0. J Neuroinflammation. 2022. PMID: 35689216 Free PMC article.
-
Cyclic Guanosine Monophosphate-Adenosine Monophosphate Synthase (cGAS), a Multifaceted Platform of Intracellular DNA Sensing.Front Immunol. 2021 Feb 23;12:637399. doi: 10.3389/fimmu.2021.637399. eCollection 2021. Front Immunol. 2021. PMID: 33708225 Free PMC article. Review.
-
Mitochondrial DNA drives noncanonical inflammation activation via cGAS-STING signaling pathway in retinal microvascular endothelial cells.Cell Commun Signal. 2020 Oct 28;18(1):172. doi: 10.1186/s12964-020-00637-3. Cell Commun Signal. 2020. PMID: 33115500 Free PMC article.
-
Inhibition of inflammation and apoptosis through the cyclic GMP-AMP synthase-stimulator of interferon genes pathway by stress granules after ALKBH5 demethylase activation during diabetic myocardial ischaemia-reperfusion injury.Diabetes Obes Metab. 2024 Sep;26(9):3940-3957. doi: 10.1111/dom.15743. Epub 2024 Jul 11. Diabetes Obes Metab. 2024. PMID: 38988216
-
Molecular mechanisms and cellular functions of cGAS-STING signalling.Nat Rev Mol Cell Biol. 2020 Sep;21(9):501-521. doi: 10.1038/s41580-020-0244-x. Epub 2020 May 18. Nat Rev Mol Cell Biol. 2020. PMID: 32424334 Review.
Cited by
-
Inhibition of cGAS attenuates neonatal hypoxic-ischemic encephalopathy via regulating microglia polarization and pyroptosis.Transl Pediatr. 2024 Aug 31;13(8):1378-1394. doi: 10.21037/tp-24-148. Epub 2024 Aug 28. Transl Pediatr. 2024. PMID: 39263289 Free PMC article.
-
The Dual Role of Microglia in Blood-Brain Barrier Dysfunction after Stroke.Curr Neuropharmacol. 2020;18(12):1237-1249. doi: 10.2174/1570159X18666200529150907. Curr Neuropharmacol. 2020. PMID: 32469699 Free PMC article. Review.
-
Structural dissection of sequence recognition and catalytic mechanism of human LINE-1 endonuclease.Nucleic Acids Res. 2021 Nov 8;49(19):11350-11366. doi: 10.1093/nar/gkab826. Nucleic Acids Res. 2021. PMID: 34554261 Free PMC article.
-
RORα-activated mitophagy attenuating hypoxic-ischemic encephalopathy via suppression of microglial cGAS-STING axis.Front Immunol. 2025 Jul 29;16:1592737. doi: 10.3389/fimmu.2025.1592737. eCollection 2025. Front Immunol. 2025. PMID: 40799648 Free PMC article.
-
Advancement of epigenetics in stroke.Front Neurosci. 2022 Oct 13;16:981726. doi: 10.3389/fnins.2022.981726. eCollection 2022. Front Neurosci. 2022. PMID: 36312038 Free PMC article. Review.
References
-
- Vannucci RC and Vannucci SJ, A model of perinatal hypoxic-ischemic brain damage. Ann N Y Acad Sci, 1997. 835: p. 234–49. - PubMed
-
- Volpe JJ: Perinatal brain injury: from pathogenesis to neuroprotection. Ment Retard Dev Disabil Res Rev 2001, 7:56–64. - PubMed
-
- Shetty J: Neonatal seizures in hypoxic-ischaemic encephalopathy--risks and benefits of anticonvulsant therapy. Dev Med Child Neurol 2015, 57 Suppl 3:40–43. - PubMed
-
- Gill MB, Perez-Polo JR: Hypoxia ischemia-mediated cell death in neonatal rat brain. Neurochem Res 2008, 33:2379–2389. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

