Vessel graft fabricated by the on-site differentiation of human mesenchymal stem cells towards vascular cells on vascular extracellular matrix scaffold under mechanical stimulation in a rotary bioreactor
- PMID: 32255003
- PMCID: PMC11299192
- DOI: 10.1039/c8tb03348j
Vessel graft fabricated by the on-site differentiation of human mesenchymal stem cells towards vascular cells on vascular extracellular matrix scaffold under mechanical stimulation in a rotary bioreactor
Abstract
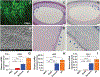
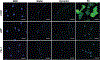
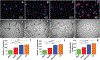
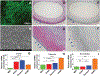
Although a significant number of studies on vascular tissue engineering have been reported, the current availability of vessel substitutes in the clinic remains limited mainly due to the mismatch of their mechanical properties and biological functions with native vessels. In this study, a novel approach to fabricating a vessel graft for vascular tissue engineering was developed by promoting differentiation of human bone marrow mesenchymal stem cells (MSCs) into endothelial cells (ECs) and vascular smooth muscle cells (VSMCs) on a native vascular extracellular matrix (ECM) scaffold in a rotary bioreactor. The expression levels of CD31 and vWF, and the LDL uptake capacity as well as the angiogenesis capability of the EC-like cells in the dynamic culture system were significantly enhanced compared to the static system. In addition, α-actin and smoothelin expression, and contractility of VSMC-like cells harvested from the dynamic model were much higher than those in a static culture system. The combination of on-site differentiation of stem cells towards vascular cells in the natural vessel ECM scaffold and maturation of the resulting vessel construct in a dynamic cell culture environment provides a promising approach to fabricating a clinically applicable vessel graft with similar mechanical properties and physiological functions to those of native blood vessels.
Conflict of interest statement
Conflicts of interest
There are no conflicts to declare.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

