Human Lung Conventional Dendritic Cells Orchestrate Lymphoid Neogenesis during Chronic Obstructive Pulmonary Disease
- PMID: 32255375
- PMCID: PMC7616955
- DOI: 10.1164/rccm.201906-1123OC
Human Lung Conventional Dendritic Cells Orchestrate Lymphoid Neogenesis during Chronic Obstructive Pulmonary Disease
Abstract
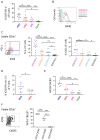
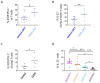
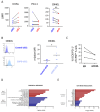
Rationale: Emerging evidence supports a crucial role for tertiary lymphoid organs (TLOs) in chronic obstructive pulmonary disease (COPD) progression. However, mechanisms of immune cell activation leading to TLOs in COPD remain to be defined.Objectives: To examine the role of lung dendritic cells (DCs) in T follicular helper (Tfh)-cell induction, a T-cell subset critically implicated in lymphoid organ formation, in COPD.Methods: Myeloid cell heterogeneity and phenotype were studied in an unbiased manner via single-cell RNA sequencing on HLA-DR+ cells sorted from human lungs. We measured the in vitro capability of control and COPD lung DC subsets, sorted using a fluorescence-activated cell sorter, to polarize IL-21+CXCL13+ (IL-21-positive and C-X-C chemokine ligand type 13-positive) Tfh-like cells. In situ imaging analysis was performed on Global Initiative for Chronic Obstructive Lung Disease stage IV COPD lungs with TLOs.Measurements and Main Results: Single-cell RNA-sequencing analysis revealed a high degree of heterogeneity among human lung myeloid cells. Among these, conventional dendritic type 2 cells (cDC2s) showed increased induction of IL-21+CXCL13+ Tfh-like cells. Importantly, the capacity to induce IL-21+ Tfh-like cells was higher in cDC2s from patients with COPD than in those from control patients. Increased Tfh-cell induction by COPD cDC2s correlated with increased presence of Tfh-like cells in COPD lungs as compared with those in control lungs, and cDC2s colocalized with Tfh-like cells in TLOs of COPD lungs. Mechanistically, cDC2s exhibited a unique migratory signature and (transcriptional) expression of several pathways and genes related to DC-induced Tfh-cell priming. Importantly, blocking the costimulatory OX40L (OX40 ligand)-OX40 axis reduced Tfh-cell induction by control lung cDC2s.Conclusions: In COPD lungs, we found lung EBI2+ (Epstein-Barr virus-induced gene 2-positive) OX-40L-expressing cDC2s that induced IL-21+ Tfh-like cells, suggesting an involvement of these cells in TLO formation.
Keywords: T follicular helper cells; chronic obstructive pulmonary disease (COPD); dendritic cells; tertiary lymphoid organs.
Figures




Comment in
-
It's Complicated: Lung Dendritic Cells in Chronic Obstructive Pulmonary Disease.Am J Respir Crit Care Med. 2020 Aug 15;202(4):479-481. doi: 10.1164/rccm.202004-0899ED. Am J Respir Crit Care Med. 2020. PMID: 32286855 Free PMC article. No abstract available.
References
-
- Hogg JC, Chu F, Utokaparch S, Woods R, Elliott WM, Buzatu L, Cherniack RM, Rogers RM, Sciurba FC, Coxson HO, Pare PD. The nature of small-airway obstruction in chronic obstructive pulmonary disease. N Engl J Med. 2004;350:2645–2653. - PubMed
-
- Ladjemi MZ, Martin C, Lecocq M, Detry B, Aboubakar Nana F, Moulin C, Weynand B, Fregimilicka C, Bouzin C, Thurion P, Carlier F, et al. Increased IgA Expression in Lung Lymphoid Follicles in Severe COPD. Am J Respir Crit Care Med. 2018 - PubMed
-
- Polverino F, Cosio BG, Pons J, Laucho-Contreras M, Tejera P, Iglesias A, Rios A, Jahn A, Sauleda J, Divo M, Pinto-Plata V, et al. B Cell-Activating Factor. An Orchestrator of Lymphoid Follicles in Severe Chronic Obstructive Pulmonary Disease. Am J Respir Crit Care Med. 2015;192:695–705. doi: 10.1164/rccm.201501-0107OC. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

