Evolution of multifunctionality through a pleiotropic substitution in the innate immune protein S100A9
- PMID: 32255429
- PMCID: PMC7213983
- DOI: 10.7554/eLife.54100
Evolution of multifunctionality through a pleiotropic substitution in the innate immune protein S100A9
Abstract
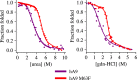
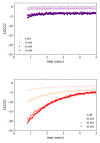
Multifunctional proteins are evolutionary puzzles: how do proteins evolve to satisfy multiple functional constraints? S100A9 is one such multifunctional protein. It potently amplifies inflammation via Toll-like receptor four and is antimicrobial as part of a heterocomplex with S100A8. These two functions are seemingly regulated by proteolysis: S100A9 is readily degraded, while S100A8/S100A9 is resistant. We take an evolutionary biochemical approach to show that S100A9 evolved both functions and lost proteolytic resistance from a weakly proinflammatory, proteolytically resistant amniote ancestor. We identify a historical substitution that has pleiotropic effects on S100A9 proinflammatory activity and proteolytic resistance but has little effect on S100A8/S100A9 antimicrobial activity. We thus propose that mammals evolved S100A8/S100A9 antimicrobial and S100A9 proinflammatory activities concomitantly with a proteolytic 'timer' to selectively regulate S100A9. This highlights how the same mutation can have pleiotropic effects on one functional state of a protein but not another, thus facilitating the evolution of multifunctionality.
Keywords: ancestral sequence reconstruction; antimicrobial activity; biochemistry; chemical biology; evolutionary biology; inflammation; mammals; none; pleiotropy; protein evolution.
Plain language summary
A single protein sometimes does multiple jobs. For instance, our immune system uses a small number of multipurpose proteins to respond quickly to a large number of threats. One example is the protein S100A9. It acts as an antimicrobial by preventing microbes from getting the nutrients they need, while also stimulating inflammation by inducing the release of molecules that recruit white blood cells. S100A9, like all proteins, is made up of a chain of small building blocks. These building blocks interact with each other and with other molecules in the environment. The sequence of the building blocks thus determines what jobs the protein can do. Therefore, a single change to the sequence of building blocks can have a dramatic effect: one change might render the protein faulty, while another change might allow it to do a new job. Proteins face similar challenges humans do when trying to do several things at once. A person driving a car while using their phone will not do either task well. Likewise, a protein that does two jobs faces challenges a single-purpose protein does not. Harman et al. were interested in how S100A9 was able to evolve and maintain its dual functionality, despite this potential problem. They started by asking when S100A9 acquired its two purposes. They measured the antimicrobial and inflammatory activity of S100A9 proteins from humans, mice and opossums. The activities of S100A9 in these species was similar, suggesting that S100A9 acquired its different jobs in the ancestor of mammals, some 160 million years ago. Next, Harman et al. computationally reconstructed ancestral forms of S100A9 by comparing hundreds of similar proteins and building an evolutionary tree. They then measured the antimicrobial and inflammatory activity of these ancestral proteins. By comparing the last ancestor that did not have these activities to the first ancestor that did, they identified the sequence changes that gave S100A9 its dual activity. Importantly, these changes are located in separate regions of the protein, meaning they could occur independently, without affecting each other. Further, the same sequence change that converted S100A9 into an inflammatory signal also introduced a mechanism to regulate this activity. The results suggest that a small number of sequence changes – or even a single change – can make a protein more versatile. This means that evolving multipurpose proteins may not be as difficult as is often thought.
© 2020, Harman et al.
Conflict of interest statement
JH, AL, GW, MH, KL, MH No competing interests declared
Figures


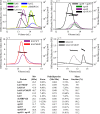
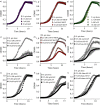

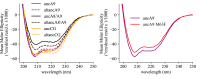
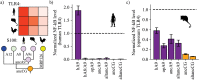
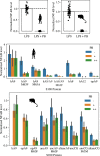

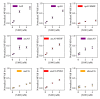


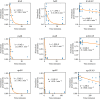
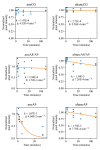
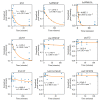
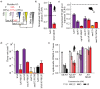

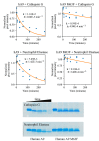
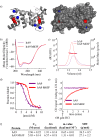

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

