Learning from amyloid trials in Alzheimer's disease. A virtual patient analysis using a quantitative systems pharmacology approach
- PMID: 32255562
- PMCID: PMC7983876
- DOI: 10.1002/alz.12082
Learning from amyloid trials in Alzheimer's disease. A virtual patient analysis using a quantitative systems pharmacology approach
Abstract
Background: Many trials of amyloid-modulating agents fail to improve cognitive outcome in Alzheimer's disease despite substantial reduction of amyloid β levels.
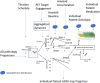
Methods: We applied a mechanism-based Quantitative Systems Pharmacology model exploring the pharmacodynamic interactions of apolipoprotein E (APOE), Catechol -O -methyl Transferase (COMTVal158Met), and 5-HT transporter (5-HTTLPR) rs25531 genotypes and aducanumab.
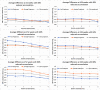
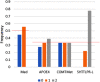
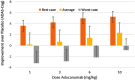
Results: The model predicts large clinical variability. Anticipated placebo differences on Alzheimer's Disease Assessment Scale (ADAS)-COG in the aducanumab ENGAGE and EMERGE ranged from 0.77 worsening to 1.56 points improvement, depending on the genotype-comedication combination. 5-HTTLPR L/L subjects are found to be the most resilient. Virtual patient simulations suggest improvements over placebo between 4% and 20% at the 10 mg/kg dose, depending on the imbalance of the 5-HTTLPR genotype and exposure. In the Phase II PRIME trial, maximal anticipated placebo difference at 10 mg/kg ranges from 0.3 worsening to 5.3 points improvement.
Discussion: These virtual patient simulations, once validated against clinical data, could lead to better informed future clinical trial designs.
Keywords: aducanumab; genotype; medication; pharmacodynamic effect; responder profile.
© 2020 In Silico Biosciences. Alzheimer's & Dementia: The Journal of the Alzheimer's Association published by Wiley Periodicals LLC on behalf of the Alzheimer's Association.
Conflict of interest statement
At the time of the study, the authors were employees of In Silico Biosciences. HG is now with Certara‐QSP.
Figures

References
-
- Foroutan N, Hopkins RB, Tarride JE, Florez ID, Levine M. Safety and efficacy of active and passive immunotherapy in mild‐to‐moderate Alzheimer's disease: A systematic review and network meta‐analysis. Clin Invest Med. 2019;42:E53‐E65. - PubMed
-
- Penninkilampi R, Brothers HM, Eslick GD. Safety and Efficacy of Anti‐Amyloid‐beta immunotherapy in Alzheimer's disease: a systematic review and meta‐analysis. J Neuroimmune Pharmacol. 2017;12:194‐203. - PubMed
-
- Aisen PS, Siemers E, Michelson D, et al. What have we learned from expedition III and EPOCH trials? Perspective of the CTAD task force. J Prev Alzheimers Dis. 2018;5:171‐174. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

