T follicular regulatory cells and IL-10 promote food antigen-specific IgE
- PMID: 32255767
- PMCID: PMC7324176
- DOI: 10.1172/JCI132249
T follicular regulatory cells and IL-10 promote food antigen-specific IgE
Abstract
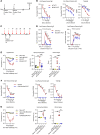
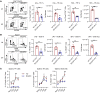
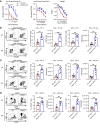
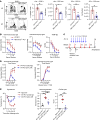
Food allergies are a major clinical problem and are driven by IgE antibodies (Abs) specific for food antigens (Ags). T follicular regulatory (Tfr) cells are a specialized subset of FOXP3+ T cells that modulate Ab responses. Here, we analyzed the role of Tfr cells in regulating Ag-specific IgE using a peanut-based food allergy model in mice. Peanut-specific IgE titers and anaphylaxis responses were significantly blunted in Tfr cell-deficient Foxp3-Cre Bcl6fl/fl mice. Loss of Tfr cells led to greatly increased nonspecific IgE levels, showing that Tfr cells have both helper and suppressor functions in IgE production in the germinal center (GC) that work together to facilitate the production of Ag-specific IgE. Foxp3-Cre Ptenfl/fl mice with augmented Tfr cell responses had markedly higher levels of peanut-specific IgE, revealing an active helper function by Tfr cells on Ag-specific IgE. The helper function of Tfr cells for IgE production involves IL-10, and the loss of IL-10 signaling by B cells led to a severely curtailed peanut-specific IgE response, decreased GCB cell survival, and loss of GC dark zone B cells after peanut sensitization. We thus reveal that Tfr cells have an unexpected helper role in promoting food allergy and may represent a target for drug development.
Keywords: Adaptive immunity; Allergy; Immunology.
Conflict of interest statement
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

