The Exercise Training Modulatory Effects on the Obesity-Induced Immunometabolic Dysfunctions
- PMID: 32256095
- PMCID: PMC7090203
- DOI: 10.2147/DMSO.S234992
The Exercise Training Modulatory Effects on the Obesity-Induced Immunometabolic Dysfunctions
Abstract
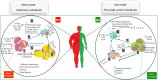
Reduced physical activity rate in people's lifestyle is a global concern associated with the prevalence of health disorders such as obesity and metabolic disturbance. Ample evidence has indicated a critical role of the immune system in the aggravation of obesity. The type, duration, and production of adipose tissue-released mediators may change subsequent inactive lifestyle-induced obesity, leading to the chronic systematic inflammation and monocyte/macrophage (MON/MФ) phenotype polarization. Preliminary adipose tissue expansion can be inhibited by changing the lifestyle. In this context, exercise training is widely recommended due to a definite improvement of energy balance and the potential impacts on the inflammatory signaling cascades. How exercise training affects the immune system has not yet been fully elucidated, because its anti-inflammatory, pro-inflammatory, or even immunosuppressive impacts have been indicated in the literature. A thorough understanding of the mechanisms triggered by exercise can suggest a new approach to combat meta-inflammation-induced metabolic diseases. In this review, we summarized the obesity-induced inflammatory pathways, the roles of MON/MФ polarization in adipose tissue and systemic inflammation, and the underlying inflammatory mechanisms triggered by exercise during obesity.
Keywords: adipose tissue; exercise training; immune system; macrophage polarization; meta-inflammation; obesity; toll-like receptors.
© 2020 Soltani et al.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Figures

References
Publication types
LinkOut - more resources
Full Text Sources

