Detection of PD-L1 Expression and Its Clinical Significance in Circulating Tumor Cells from Patients with Non-Small-Cell Lung Cancer
- PMID: 32256114
- PMCID: PMC7093656
- DOI: 10.2147/CMAR.S245425
Detection of PD-L1 Expression and Its Clinical Significance in Circulating Tumor Cells from Patients with Non-Small-Cell Lung Cancer
Abstract
Background: The expression of programmed cell death ligand 1(PD-L1) is related to the efficacy of immune checkpoint inhibitors on patients with non-small cell lung cancer (NSCLC), but tumor tissue (TT) samples are difficult to obtain, and initial TT samples are difficult to reflect the spatial-temporal heterogeneity. Therefore, we explored the feasibility of separating circulating tumor cells (CTCs) and detecting PD-L1 expression on CTCs.
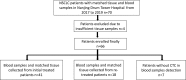
Patients and methods: Peripheral blood specimens were sampled from 66 NSCLC patients, and CTCs were separated by membrane filtration based on size. For 59 patients with paired TT specimens, the expression of PD-L1 in their CTCs and TTs was determined using the immunohistochemistry and immunocytochemistry based on 28-8 antibody, respectively. The PD-L1 expression in TTs was set as a gold standard for calculation of sensitivity, specificity, consistency, positive predictive value (PPV), and negative predictive value (NPV), and the Cohen kappa coefficient for CTCs and paired TTs was calculated. In addition, the T-test, Chi-square test, and Mann-Whitney U-test were adopted to analyze the correlation of clinical pathological features and prognosis with PD-L1 expression.
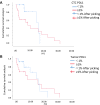
Results: Sensitivity, specificity, concordance, PPV and NPV of detecting PD-L1 in CTCs of the 41 initial treated patients were 88.89%, 73.91%, 80%, 72.73% and 89.47%, respectively, and the Cohen kappa coefficient of CTC and paired TTs was 0.613. The univariate analysis of survival showed that the progression-free survival time of initial treated patients with positive PD-L1 expression was shorter than that of those with negative PD-L1 expression in CTCs or TTs (P>0.05), and the positive PD-L1 expression in CTCs or TTs had nothing to do with age, sex, smoking status, histological type, and stage (P > 0.05).
Conclusion: The study confirms the feasibility of CTC PD-L1 detection in peripheral blood and lays a foundation for exploring real-time and individualized immunotherapy molecular biomarkers.
Keywords: PD-L1 level/expression; circulating tumor cell; immunotherapy; non-small cell lung cancer.
© 2020 Cheng et al.
Conflict of interest statement
The authors report no conflicts of interest in this work.
Figures


Similar articles
-
PD-L1 expression in circulating tumor cells of advanced non-small cell lung cancer patients treated with nivolumab.Lung Cancer. 2018 Jun;120:108-112. doi: 10.1016/j.lungcan.2018.04.001. Epub 2018 Apr 3. Lung Cancer. 2018. PMID: 29748004
-
Circulating tumor cells PD-L1 expression detection and correlation of therapeutic efficacy of immune checkpoint inhibition in advanced non-small-cell lung cancer.Thorac Cancer. 2023 Feb;14(5):470-478. doi: 10.1111/1759-7714.14767. Epub 2023 Jan 11. Thorac Cancer. 2023. PMID: 36630992 Free PMC article.
-
Detection of PD-L1 in circulating tumor cells and white blood cells from patients with advanced non-small-cell lung cancer.Ann Oncol. 2018 Jan 1;29(1):193-199. doi: 10.1093/annonc/mdx636. Ann Oncol. 2018. PMID: 29361135
-
Prognostic significance of programmed cell death-ligand 1 expression on circulating tumor cells in various cancers: A systematic review and meta-analysis.Cancer Med. 2021 Oct;10(20):7021-7039. doi: 10.1002/cam4.4236. Epub 2021 Aug 23. Cancer Med. 2021. PMID: 34423578 Free PMC article.
-
Circulating Tumor Cell PD-L1 Expression as Biomarker for Therapeutic Efficacy of Immune Checkpoint Inhibition in NSCLC.Cells. 2019 Aug 1;8(8):809. doi: 10.3390/cells8080809. Cells. 2019. PMID: 31374957 Free PMC article. Review.
Cited by
-
The Clinical Significance of Deglycosylated PD-L1 Level Detection Using 28-8 Monoclonal Antibody in Lung Adenocarcinoma.Int J Gen Med. 2022 Sep 19;15:7383-7393. doi: 10.2147/IJGM.S381530. eCollection 2022. Int J Gen Med. 2022. PMID: 36164284 Free PMC article.
-
Design and Performance Analysis of Spiral Microchannels for Efficient Particle Separation Using Inertial Microfluidics.Micromachines (Basel). 2025 Mar 19;16(3):349. doi: 10.3390/mi16030349. Micromachines (Basel). 2025. PMID: 40141959 Free PMC article.
-
Analysis of DNA from liquid biopsy: new genetic biomarkers for cancer immunotherapy?Explor Target Antitumor Ther. 2021;2(2):204-207. doi: 10.37349/etat.2021.00041. Epub 2021 Apr 30. Explor Target Antitumor Ther. 2021. PMID: 36046146 Free PMC article. No abstract available.
-
PD-L1/pS6 in Circulating Tumor Cells (CTCs) during Osimertinib Treatment in Patients with Non-Small Cell Lung Cancer (NSCLC).Biomedicines. 2022 Aug 5;10(8):1893. doi: 10.3390/biomedicines10081893. Biomedicines. 2022. PMID: 36009440 Free PMC article.
-
Correlation between PD-L1 expression ON CTCs and prognosis of patients with cancer: a systematic review and meta-analysis.Oncoimmunology. 2021 Jun 21;10(1):1938476. doi: 10.1080/2162402X.2021.1938476. Oncoimmunology. 2021. PMID: 34211802 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

