Reversible inhibitor of CRM1 sensitizes glioblastoma cells to radiation by blocking the NF-κB signaling pathway
- PMID: 32256206
- PMCID: PMC7106748
- DOI: 10.1186/s12935-020-01186-y
Reversible inhibitor of CRM1 sensitizes glioblastoma cells to radiation by blocking the NF-κB signaling pathway
Retraction in
-
Retraction Note: Reversible inhibitor of CRM1 sensitizes glioblastoma cells to radiation by blocking the NF-κB signaling pathway.Cancer Cell Int. 2024 Sep 18;24(1):320. doi: 10.1186/s12935-024-03508-w. Cancer Cell Int. 2024. PMID: 39294636 Free PMC article. No abstract available.
Abstract
Background: Activation of nuclear factor-kappa B (NF-κΒ) through DNA damage is one of the causes of tumor cell resistance to radiotherapy. Chromosome region 1 (CRM1) regulates tumor cell proliferation, drug resistance, and radiation resistance by regulating the nuclear-cytoplasmic translocation of important tumor suppressor proteins or proto-oncoproteins. A large number of studies have reported that inhibition of CRM1 suppresses the activation of NF-κΒ. Thus, we hypothesize that the reversible CRM1 inhibitor S109 may induce radiosensitivity in glioblastoma (GBM) by regulating the NF-κΒ signaling pathway.
Methods: This study utilized the cell counting kit-8 (CCK-8), 5-ethynyl-2'-deoxyuridine (EdU), and colony formation assay to evaluate the effect of S109 combined with radiotherapy on the proliferation and survival of GBM cells. The therapeutic efficacy of S109 combined with radiotherapy was evaluated in vivo to explore the therapeutic mechanism of S109-induced GBM radiosensitization.
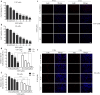
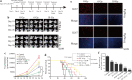
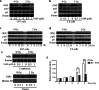
Results: We found that S109 combined with radiotherapy significantly inhibited GBM cell proliferation and colony formation. By regulating the levels of multiple cell cycle- and apoptosis-related proteins, the combination therapy induced G1 cell cycle arrest in GBM cells. In vivo studies showed that S109 combined with radiotherapy significantly inhibited the growth of intracranial GBM and prolonged survival. Importantly, we found that S109 combined with radiotherapy promoted the nuclear accumulation of IκΒα, and inhibited phosphorylation of p65 and the transcriptional activation of NF-κΒ.
Conclusion: Our findings provide a new therapeutic regimen for improving GBM radiosensitivity as well as a scientific basis for further clinical trials to evaluate this combination therapy.
Keywords: CRM1; GBM; Irradiation; NF-κΒ signaling pathway; S109.
© The Author(s) 2020.
Conflict of interest statement
Competing interestsThe authors declare that they have no competing interests.
Figures


References
-
- Qu DW, Liu Y, Wang L, Xiong Y, Zhang CL, Gao DS. Glial cell line-derived neurotrophic factor promotes proliferation of neuroglioma cells by up-regulation of cyclins PCNA and Ki-67. Eur Rev Med Pharmacol Sci. 2015;19(11):2070–5. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

