Circular RNA circVAPA knockdown suppresses colorectal cancer cell growth process by regulating miR-125a/CREB5 axis
- PMID: 32256212
- PMCID: PMC7106619
- DOI: 10.1186/s12935-020-01178-y
Circular RNA circVAPA knockdown suppresses colorectal cancer cell growth process by regulating miR-125a/CREB5 axis
Abstract
Background: Colorectal cancer (CRC) is a malignant tumor, and the overall prognosis of patients with advanced CRC is still unsatisfactory. Circular RNAs (circRNAs) vesicle-associated membrane protein-associated protein A (circVAPA) could act as an underlying biomarker in CRC. This study aimed to explore the mechanism of circVAPA in the regulation of CRC growth.
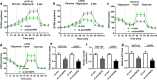
Methods: CircVAPA level was measured in CRC tumor tissues. The expression levels of circVAPA, VAPA mRNA, microRNA-125a (miR-125a), and cAMP response element binding 5 (CREB5) in CRC cells were detected by RT-qPCR. Cell cycle progression, migration and invasion, extracellular acidification rate (ECAR) and oxygen consumption rate (OCR) were measured by flow cytometry, transwell assays and Seahorse XF96 Glycolysis Analyzer, severally. The levels of glucose uptake, lactate and ATP production were examined by Glucose Uptake Colorimetric Assay kit, Lactate Assay kit and ATP Colorimetric Assay kit, respectively. The interaction between miR-125a and circVAPA or CREB5 was predicted by Starbase or DIANA TOOL, and verified by the dual-luciferase reporter and RNA Immunoprecipitation (RIP) assays.
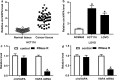
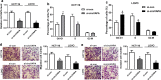
Results: CircVAPA level was up-regulated in CRC tumor tissues. Expression levels of circVAPA and CREB5 were increased, and miR-125a was decreased in CRC cells. CircVAPA knockdown repressed CRC cells cycle progression, migration, invasion and glycolysis. CircVAPA acted as a miR-125a sponge to regulate CREB5 expression. Rescue assay confirmed that miR-125a deletion or CREB5 overexpression weakened the inhibitory effect of circVAPA knockdown on CRC growth.
Conclusion: Our studies disclosed that circVAPA knockdown suppressed CRC cells cycle progression, migration, invasion and glycolysis partly by modulating miR-125a/CREB5 axis, suggesting a potential therapeutic strategy for CRC treatment.
Keywords: CREB5; CircVAPA; Colorectal cancer; miR-125a.
© The Author(s) 2020.
Conflict of interest statement
Competing interestsThe authors declare that they have no financial conflicts of interest.
Figures



Similar articles
-
Circular RNA circVAPA is up-regulated and exerts oncogenic properties by sponging miR-101 in colorectal cancer.Biomed Pharmacother. 2019 Apr;112:108611. doi: 10.1016/j.biopha.2019.108611. Epub 2019 Feb 21. Biomed Pharmacother. 2019. PMID: 30797148
-
Circular RNA circVAPA contributes to non-small-cell lung cancer progression via miR-342-3p-dependent regulation of ZEB2.World J Surg Oncol. 2021 Nov 29;19(1):335. doi: 10.1186/s12957-021-02447-4. World J Surg Oncol. 2021. PMID: 34839824 Free PMC article.
-
CircTADA2A suppresses the progression of colorectal cancer via miR-374a-3p/KLF14 axis.J Exp Clin Cancer Res. 2020 Aug 15;39(1):160. doi: 10.1186/s13046-020-01642-7. J Exp Clin Cancer Res. 2020. PMID: 32799891 Free PMC article.
-
circVAPA promotes the proliferation, migration and invasion of oral cancer cells through the miR-132/HOXA7 axis.J Int Med Res. 2021 Jun;49(6):3000605211013207. doi: 10.1177/03000605211013207. J Int Med Res. 2021. PMID: 34102907 Free PMC article.
-
LncRNA SNHG5 affects cell proliferation, metastasis and migration of colorectal cancer through regulating miR-132-3p/CREB5.Cancer Biol Ther. 2019;20(4):524-536. doi: 10.1080/15384047.2018.1537579. Epub 2018 Nov 5. Cancer Biol Ther. 2019. PMID: 30395767 Free PMC article.
Cited by
-
The ATF2/miR-3913-5p/CREB5 axis is involved in the cell proliferation and metastasis of colorectal cancer.Commun Biol. 2023 Oct 10;6(1):1026. doi: 10.1038/s42003-023-05405-w. Commun Biol. 2023. PMID: 37816820 Free PMC article.
-
Exploring the Key Signaling Pathways and ncRNAs in Colorectal Cancer.Int J Mol Sci. 2024 Apr 21;25(8):4548. doi: 10.3390/ijms25084548. Int J Mol Sci. 2024. PMID: 38674135 Free PMC article. Review.
-
Oncogenic Functions and Clinical Significance of Circular RNAs in Colorectal Cancer.Cancers (Basel). 2021 Jul 6;13(14):3395. doi: 10.3390/cancers13143395. Cancers (Basel). 2021. PMID: 34298612 Free PMC article. Review.
-
Circ-PRKDC Facilitates the Progression of Colorectal Cancer Through miR-198/DDR1 Regulatory Axis.Cancer Manag Res. 2020 Dec 15;12:12853-12865. doi: 10.2147/CMAR.S273484. eCollection 2020. Cancer Manag Res. 2020. PMID: 33364834 Free PMC article.
-
miRNA Clusters with Down-Regulated Expression in Human Colorectal Cancer and Their Regulation.Int J Mol Sci. 2020 Jun 29;21(13):4633. doi: 10.3390/ijms21134633. Int J Mol Sci. 2020. PMID: 32610706 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Miscellaneous

