Multiplexed 129Xe HyperCEST MRI Detection of Genetically Reconstituted Bacterial Protein Nanoparticles in Human Cancer Cells
- PMID: 32256252
- PMCID: PMC7091528
- DOI: 10.1155/2020/5425934
Multiplexed 129Xe HyperCEST MRI Detection of Genetically Reconstituted Bacterial Protein Nanoparticles in Human Cancer Cells
Abstract
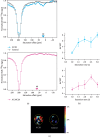
Gas vesicle nanoparticles (GVs) are gas-containing protein assemblies expressed in bacteria and archaea. Recently, GVs have gained considerable attention for biotechnological applications as genetically encodable contrast agents for MRI and ultrasonography. However, at present, the practical use of GVs is hampered by a lack of robust methodology for their induction into mammalian cells. Here, we demonstrate the genetic reconstitution of protein nanoparticles with characteristic bicone structures similar to natural GVs in a human breast cancer cell line KPL-4 and genetic control of their size and shape through expression of reduced sets of humanized gas vesicle genes cloned into Tol2 transposon vectors, referencing the natural gas vesicle gene clusters of the cyanobacteria planktothrix rubescens/agardhii. We then report the utility of these nanoparticles as multiplexed, sensitive, and genetically encoded contrast agents for hyperpolarized xenon chemical exchange saturation transfer (HyperCEST) MRI.
Copyright © 2020 Ryota Mizushima et al.
Conflict of interest statement
RIKEN has a pending patent application already open to the public (WO2018043716A1) regarding this work, in which R. M. and T. M. W. are inventors.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
