GSK3β and Tau Protein in Alzheimer's Disease and Epilepsy
- PMID: 32256316
- PMCID: PMC7089874
- DOI: 10.3389/fncel.2020.00019
GSK3β and Tau Protein in Alzheimer's Disease and Epilepsy
Abstract
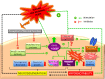
Alzheimer's disease (AD) is the most common form of dementia present in older adults; its etiology involves genetic and environmental factors. In recent years, epidemiological studies have shown a correlation between AD and chronic epilepsy since a considerable number of patients with AD may present seizures later on. Although the pathophysiology of seizures in AD is not completely understood, it could represent the result of several molecular mechanisms linked to amyloid beta-peptide (Aβ) accumulation and the hyperphosphorylation of tau protein, which may induce an imbalance in the release and recapture of excitatory and inhibitory neurotransmitters, structural alterations of the neuronal cytoskeleton, synaptic loss, and neuroinflammation. These changes could favor the recurrent development of hypersynchronous discharges and epileptogenesis, which, in a chronic state, favor the neurodegenerative process and influence the cognitive decline observed in AD. Supporting this correlation, histopathological studies in the brain tissue of temporal lobe epilepsy (TLE) patients have revealed the presence of Aβ deposits and the accumulation of tau protein in the neurofibrillary tangles (NFTs), accompanied by an increase of glycogen synthase kinase-3 beta (GSK3β) activity that may lead to an imminent alteration in posttranslational modifications of some microtubule-associated proteins (MAPs), mainly tau. The present review is focused on understanding the pathological aspects of GSK3β and tau in the development of TLE and AD.
Keywords: GSK3β; epilepsy; hippocampal sclerosis; neurodegeneration; tau protein.
Copyright © 2020 Toral-Rios, Pichardo-Rojas, Alonso-Vanegas and Campos-Peña.
Figures



References
-
- Ahnaou A., Moechars D., Raeymaekers L., Biermans R., Manyakov N. V., Bottelbergs A., et al. (2017). Emergence of early alterations in network oscillations and functional connectivity in a tau seeding mouse model of Alzheimer’s disease pathology. Sci. Rep. 7:14189 10.1038/s41598-017-13839-6 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

