Transcutaneous Auricular Vagus Nerve Stimulation-Paired Rehabilitation for Oromotor Feeding Problems in Newborns: An Open-Label Pilot Study
- PMID: 32256328
- PMCID: PMC7093597
- DOI: 10.3389/fnhum.2020.00077
Transcutaneous Auricular Vagus Nerve Stimulation-Paired Rehabilitation for Oromotor Feeding Problems in Newborns: An Open-Label Pilot Study
Abstract
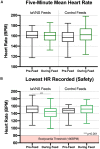
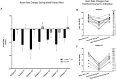
Neonates born premature or who suffer brain injury at birth often have oral feeding dysfunction and do not meet oral intake requirements needed for discharge. Low oral intake volumes result in extended stays in the hospital (>2 months) and can lead to surgical implant and explant of a gastrostomy tube (G-tube). Prior work suggests pairing vagus nerve stimulation (VNS) with motor activity accelerates functional improvements after stroke, and transcutaneous auricular VNS (taVNS) has emerged as promising noninvasive form of VNS. Pairing taVNS with bottle-feeding rehabilitation may improve oromotor coordination and lead to improved oral intake volumes, ultimately avoiding the need for G-tube placement. We investigated whether taVNS paired with oromotor rehabilitation is tolerable and safe and facilitates motor learning in infants who have failed oral feeding. We enrolled 14 infants [11 premature and 3 hypoxic-ischemic encephalopathy (HIE)] who were slated for G-tube placement in a prospective, open-label study of taVNS-paired rehabilitation to increase feeding volumes. Once-daily taVNS was delivered to the left tragus during bottle feeding for 2 weeks, with optional extension. The primary outcome was attainment of oral feeding volumes and weight gain adequate for discharge without G-tube while also monitoring discomfort and heart rate (HR) as safety outcomes. We observed no adverse events related to stimulation, and stimulation-induced HR reductions were transient and safe and likely confirmed vagal engagement. Eight of 14 participants (57%) achieved adequate feeding volumes for discharge without G-tube (mean treatment length: 16 ± 6 days). We observed significant increases in feeding volume trajectories in responders compared with pre-stimulation (p < 0.05). taVNS-paired feeding rehabilitation appears safe and may improve oral feeding in infants with oromotor dyscoordination, increasing the rate of discharge without G-tube, warranting larger controlled trials.
Keywords: feeding; hypoxic–ischemic encephalopathy; pediatric rehabilitation; transcutaneous auricular vagus nerve stimulation; transcutaneous vagus nerve stimulation; vagus nerve stimulation.
Copyright © 2020 Badran, Jenkins, Cook, Thompson, Dancy, DeVries, Mappin, Summers, Bikson and George.
Figures



References
-
- Adams-Chapman I., Bann C. M., Vaucher Y. E., Stoll B. J., Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network . (2013). Association between feeding difficulties and language delay in preterm infants using Bayley Scales of Infant Development-Third Edition. J. Pediatr. 163, 680.e1-3–685.e1-3. 10.1016/j.jpeds.2013.03.006 - DOI - PMC - PubMed
-
- Badran B. W., Dowdle L. T., Mithoefer O. J., LaBate N. T., Coatsworth J., Brown J. C., et al. . (2018a). Neurophysiologic effects of transcutaneous auricular vagus nerve stimulation (taVNS) via electrical stimulation of the tragus: a concurrent taVNS/fMRI study and review. Brain Stimul. 11, 492–500. 10.1016/j.brs.2017.12.009 - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

