Hepatoprotective Effect of Cranberry Nutraceutical Extract in Non-alcoholic Fatty Liver Model in Rats: Impact on Insulin Resistance and Nrf-2 Expression
- PMID: 32256346
- PMCID: PMC7093716
- DOI: 10.3389/fphar.2020.00218
Hepatoprotective Effect of Cranberry Nutraceutical Extract in Non-alcoholic Fatty Liver Model in Rats: Impact on Insulin Resistance and Nrf-2 Expression
Abstract
Background: Non-alcoholic fatty liver disease (NAFLD) is a pathological accumulation of triglycerides (TGs) in the hepatocyte in the absence of alcohol intake. Untreated NAFLD is expected to progress into liver fibrosis. Cranberry is rich in polyphenols with antioxidant and anti-inflammatory activities.
Hypothesis: The present study was performed to evaluate our hypothesis of the possible anti-fibrotic effect of cranberry nutraceuticals in a high fat cholesterol diet induced (HFCD)-NAFLD in rats, focusing on improving insulin sensitivity and modulating the expression of nuclear factor erythroid-2-related factor-2 (Nrf2) (a transcription factor responsible for regulating cellular redox balance).
Method: Male albino wistar rats (12 weeks) received HFCD and/or cranberry (50 and 100 mg/kg/day, three times/week) orally for 8 consecutive weeks.
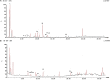
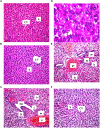
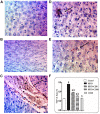
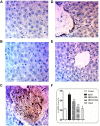
Results: In comparison to the HFCD group, cranberry treated groups (50 and 100 mg/kg) showed marked hepatoprotection, where it significantly decreased liver enzymes (alanine transaminases by 49 and 64% and aspartate transaminases by 45 and 64%; respectively), TGs, and ameliorated the histopathological alterations (such as inflammatory cells infiltration and ballooning degeneration) induced by HFCD. Cranberry also alleviated oxidative stress (malondialdehyde, glutathione, catalase and superoxide dismutase) and inflammation (tumor necrosis factor- alpha, interleukine-6 and nuclear factor kappa-b) and significantly reduced the HOMA-IR and TyG index. On the other hand, cranberry treated groups (50 and 100 mg/kg) showed a marked increase in the expression of adiponectin, by 8 and 13-fold, insulin receptor substrate-2 by 21 and 79%, and Nrf2 by 13 and 61%, respectively. Notably, cranberry significantly reduced the fibrotic markers, TGF-β and α-SMA expression and collagen deposition.
Conclusion: The present study showed that cranberry significantly attenuated NAFLD, in a dose dependent manner, which could be partially recognized by its antioxidant, anti-inflammatory activities, and its ability to improve insulin sensitivity. Notably, our study proves for the first time that the anti-fibrotic activity of cranberry is promising.
Keywords: NAFLD; Nrf-2; cranberry nutraceutical; insulin sensitivity; liver fibrosis.
Copyright © 2020 Faheem, Saeed, El-Naga, Ayoub and Azab.
Figures



References
-
- Ali H. A., Hussein M. A., Barakat M. A. (2015). Biochemical effects of cranberry extract in experimentally induced myocardial necrosis in rats. Benha Vet. Med. J. 28 155–162. 10.21608/bvmj.2015.32494 - DOI
-
- Angulo P. (2002). Nonalcoholic fatty liver disease. N. Engl. J. Med. 346 1221–1231. - PubMed
-
- Anhê F. F., Roy D., Pilon G., Dudonné S., Matamoros S., Varin T. V., et al. (2014). A polyphenol-rich cranberry extract protects from diet-induced obesity, insulin resistance and intestinal inflammation in association with increased Akkermansia spp. population in the gut microbiota of mice. Gut 64 872–883. 10.1136/gutjnl-2014-307142 - DOI - PubMed
LinkOut - more resources
Full Text Sources

