Luteolin Attenuates Allergic Nasal Inflammation via Inhibition of Interleukin-4 in an Allergic Rhinitis Mouse Model and Peripheral Blood From Human Subjects With Allergic Rhinitis
- PMID: 32256362
- PMCID: PMC7093717
- DOI: 10.3389/fphar.2020.00291
Luteolin Attenuates Allergic Nasal Inflammation via Inhibition of Interleukin-4 in an Allergic Rhinitis Mouse Model and Peripheral Blood From Human Subjects With Allergic Rhinitis
Abstract
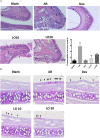
Objectives: Luteolin is the active component of Perilla frutescens, an herb for the treatment of allergy in Asia. In this study, we aimed to investigate the effects and mechanisms of luteolin treatment. Methods: BALB/c mice sensitized with house dust mite (HDM) to induce allergic rhinitis (AR), and treated with dexamethasone or luteolin. In addition, mononuclear cells from peripheral blood (PBMC) of AR patients were co-cultured with dexamethasone or luteolin, and were re-stimulated with HDM. Results: Luteolin-treated mice had decreased allergic symptoms, and serum HDM-specific IgE when compared to the untreated group. Flow cytometric analyses of splenocytes and nasal lymphoid tissues from AR mice found that luteolin decreased CD4+ IL-4-secreting T cells when compared to those from vehicle treated AR mice. Histopathology sections showed reduced infiltration of eosinophils and decreased mucus secretion of mouse nasal epithelium. In the in vitro study, the results showed that luteolin reduced the percentage of CD4+ IL-4-secreting splenocytes expression was through reducing expression of pSTAT6 and GATA3. PBMCs from AR patients pretreated with luteolin could decrease percentage of CD4+ IL-4-secreting cells. Conclusion: Our study identified that luteolin attenuates allergic nasal inflammation via inhibition of IL-4 production, which supports the potential pharmaceutical application of luteolin treatment for AR.
Keywords: IL-4; allergic rhinitis; luteolin; traditional Chinese medicine; type 2 helper T cells.
Copyright © 2020 Liang, Yu, Huang and Yen.
Figures







References
-
- Bousquet J., Khaltaev N., Cruz A. A., Denburg J., Fokkens W. J., Togias A., et al. (2008). World Health, Galen, and AllerGen, Allergic Rhinitis and its Impact on Asthma (ARIA) 2008 update (in collaboration with the World Health Organization, GA(2)LEN and AllerGen). Allergy 63, 8–160. 10.1111/j.1398-9995.2007.01620.x - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

