The Role of Candida albicans Secreted Polysaccharides in Augmenting Streptococcus mutans Adherence and Mixed Biofilm Formation: In vitro and in vivo Studies
- PMID: 32256460
- PMCID: PMC7093027
- DOI: 10.3389/fmicb.2020.00307
The Role of Candida albicans Secreted Polysaccharides in Augmenting Streptococcus mutans Adherence and Mixed Biofilm Formation: In vitro and in vivo Studies
Abstract
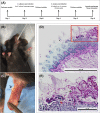
The oral cavity is a complex environment harboring diverse microbial species that often co-exist within biofilms formed on oral surfaces. Within a biofilm, inter-species interactions can be synergistic in that the presence of one organism generates a niche for another enhancing colonization. Among these species are the opportunistic fungal pathogen Candida albicans and the bacterial species Streptococcus mutans, the etiologic agents of oral candidiasis and dental caries, respectively. Recent studies have reported enhanced prevalence of C. albicans in children with caries indicating potential clinical implications for this fungal-bacterial interaction. In this study, we aimed to specifically elucidate the role of C. albicans-derived polysaccharide biofilm matrix components in augmenting S. mutans colonization and mixed biofilm formation. Comparative evaluations of single and mixed species biofilms demonstrated significantly enhanced S. mutans retention in mixed biofilms with C. albicans. Further, S. mutans single species biofilms were enhanced upon exogenous supplementation with purified matrix material derived from C. albicans biofilms. Similarly, growth in C. albicans cell-free spent biofilm culture media enhanced S. mutans single species biofilm formation, however, the observed increase in S. mutans biofilms was significantly affected upon enzymatic digestion of polysaccharides in spent media, identifying C. albicans secreted polysaccharides as a key factor in mediating mixed biofilm formation. The enhanced S. mutans biofilms mediated by the various C. albicans effectors was also demonstrated using confocal laser scanning microscopy. Importantly, a clinically relevant mouse model of oral co-infection was adapted to demonstrate the C. albicans-mediated enhanced S. mutans colonization in a host. Analyses of harvested tissue and scanning electron microscopy demonstrated significantly higher S. mutans retention on teeth and tongues of co-infected mice compared to mice infected only with S. mutans. Collectively, the findings from this study strongly indicate that the secretion of polysacharides from C. albicans in the oral environment may impact the development of S. mutans biofilms, ultimately increasing dental caries and, therefore, Candida oral colonization should be considered as a factor in evaluating the risk of caries.
Keywords: Candida albicans; Streptococcus mutans; dental caries; fungal-bacteria interactions; matrix; mixed-biofilms; polysaccharide.
Copyright © 2020 Khoury, Vila, Puthran, Sultan, Montelongo-Jauregui, Melo and Jabra-Rizk.
Figures



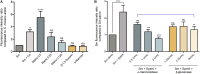




Similar articles
-
Bacterial GtfB Augments Candida albicans Accumulation in Cross-Kingdom Biofilms.J Dent Res. 2017 Sep;96(10):1129-1135. doi: 10.1177/0022034517714414. Epub 2017 Jun 12. J Dent Res. 2017. PMID: 28605597 Free PMC article.
-
Caffeic Acid Phenethyl Ester (CAPE) Inhibits Cross-Kingdom Biofilm Formation of Streptococcus mutans and Candida albicans.Microbiol Spectr. 2022 Oct 26;10(5):e0157822. doi: 10.1128/spectrum.01578-22. Epub 2022 Aug 18. Microbiol Spectr. 2022. PMID: 35980199 Free PMC article.
-
Catalase produced by Candida albicans protects Streptococcus mutans from H2O2 stress-one more piece in the cross-kingdom synergism puzzle.mSphere. 2023 Oct 24;8(5):e0029523. doi: 10.1128/msphere.00295-23. Epub 2023 Aug 21. mSphere. 2023. PMID: 37607054 Free PMC article.
-
Multifaceted roles of Candida albicans and Streptococcus mutans in contributing to polybiofilm infections in early childhood caries.Front Cell Infect Microbiol. 2025 Jun 27;15:1625103. doi: 10.3389/fcimb.2025.1625103. eCollection 2025. Front Cell Infect Microbiol. 2025. PMID: 40654570 Free PMC article. Review.
-
Oral fungal-bacterial biofilm models in vitro: a review.Med Mycol. 2018 Aug 1;56(6):653-667. doi: 10.1093/mmy/myx111. Med Mycol. 2018. PMID: 29228383 Review.
Cited by
-
Activity of Sodium Trimetaphosphate Nanoparticles on Cariogenic-Related Biofilms In Vitro.Nanomaterials (Basel). 2022 Dec 30;13(1):170. doi: 10.3390/nano13010170. Nanomaterials (Basel). 2022. PMID: 36616080 Free PMC article.
-
Peptide Designs for Use in Caries Management: A Systematic Review.Int J Mol Sci. 2023 Feb 20;24(4):4247. doi: 10.3390/ijms24044247. Int J Mol Sci. 2023. PMID: 36835657 Free PMC article.
-
The Coexistence of Bacterial Species Restructures Biofilm Architecture and Increases Tolerance to Antimicrobial Agents.Microbiol Spectr. 2023 Feb 27;11(2):e0358122. doi: 10.1128/spectrum.03581-22. Online ahead of print. Microbiol Spectr. 2023. PMID: 36847543 Free PMC article.
-
Surface adherence and vacuolar internalization of bacterial pathogens to the Candida spp. cells: Mechanism of persistence and propagation.J Adv Res. 2023 Nov;53:115-136. doi: 10.1016/j.jare.2022.12.013. Epub 2022 Dec 23. J Adv Res. 2023. PMID: 36572338 Free PMC article. Review.
-
Sodium houttuyfonate enhances the mono-therapy of fluconazole on oropharyngeal candidiasis (OPC) through HIF-1α/IL-17 axis by inhibiting cAMP mediated filamentation in Candida albicans-Candida glabrata dual biofilms.Virulence. 2022 Dec;13(1):428-443. doi: 10.1080/21505594.2022.2035066. Virulence. 2022. PMID: 35195502 Free PMC article.
References
-
- Barbieri D., Vicente V., Fraiz F., Lavoranti O., Svidzinski T., Pinheiro R. (2007). Analysis of the in vitro adherence of Streprococcus mutans and Candida albicans. Braz. J. Microbiol. 38 624–663.
Grants and funding
LinkOut - more resources
Full Text Sources

