Biotechnological and Immunological Platforms Based on PGL-I Carbohydrate-Like Peptide of Mycobacterium leprae for Antibodies Detection Among Leprosy Clinical Forms
- PMID: 32256479
- PMCID: PMC7092704
- DOI: 10.3389/fmicb.2020.00429
Biotechnological and Immunological Platforms Based on PGL-I Carbohydrate-Like Peptide of Mycobacterium leprae for Antibodies Detection Among Leprosy Clinical Forms
Abstract
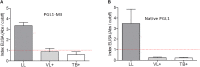
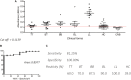
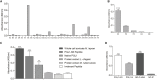
Phenolic glycolipid I (PGL-I) is an abundant antigen on the Mycobacterium leprae cell wall, commonly used for operational classification of leprosy patients. Our aim was to develop PGL-I mimotopes with similar characteristics and functions of the native antigen. We have used a random peptide phage display (PD) library for selections against the monoclonal antibody anti-PGL-I. After three selection cycles, six peptides were identified. All sequences were interspersed by a spacer generating a chimeric peptide (PGLI-M3) that was artificially synthesized. The highly reactive peptide was submitted to a reverse PD selection with a single-chain Fv (scFv) antibody fragment combinatorial library. The most reactive scFv was then validated by enzyme-linked immunosorbent assay (ELISA) against both native PGL-I and two derived synthetic (NDO and ND-O-HSA). We have further proved the scFv specificity by detecting M. leprae bacilli in leprosy lesions through immunohistochemistry. We then described its applicability in ELISA for all clinical forms and household contacts (HC). Afterward, we showed differential binding affinities of PGLI-M3 to sera (anti-PGL-I IgM) from all leprosy clinical forms through surface plasmon resonance (SPR). ELISA IgM detection showed 89.1% sensitivity and 100% specificity, considering all clinical forms. Positivity for anti-PGL-I IgM was twofold higher in both HC and patients with paucibacillary forms in hyperendemic regions than in endemic ones. The SPR immunosensor was able to differentiate clinical forms with 100% accuracy. This is the first time that a PGL-I mimotope has efficiently mimicked the carbohydrate group of the M. leprae antigen with successful immunoassay applications and may become a substitute for the native antigen.
Keywords: ELISA; mimotopes; phage display; phenolic glycolipid I; scFv; surface plasmon resonance.
Copyright © 2020 Lima, Capparelli, Dias Oliveira, Fujimura, Moraes, Araujo, Silva, Alves-Balvedi, Brito-Madurro, Goulart and Goulart.
Figures




Similar articles
-
Production and characterization of peptide mimotopes of phenolic glycolipid-I of Mycobacterium leprae.FEMS Immunol Med Microbiol. 2004 May 1;41(1):51-7. doi: 10.1016/j.femsim.2004.01.001. FEMS Immunol Med Microbiol. 2004. PMID: 15094167
-
Specific anti-M leprae PGL-I antibodies and Mitsuda reaction in the management of household contacts in New Caledonia.Int J Lepr Other Mycobact Dis. 1989 Dec;57(4):794-800. Int J Lepr Other Mycobact Dis. 1989. PMID: 2681462
-
Comparison of three immunological tests for leprosy diagnosis and detection of subclinical infection.Lepr Rev. 2011 Dec;82(4):389-401. Lepr Rev. 2011. PMID: 22439279
-
Accuracy of Enzyme-Linked Immunosorbent Assays (ELISAs) in Detecting Antibodies against Mycobacterium leprae in Leprosy Patients: A Systematic Review and Meta-Analysis.Can J Infect Dis Med Microbiol. 2018 Nov 25;2018:9828023. doi: 10.1155/2018/9828023. eCollection 2018. Can J Infect Dis Med Microbiol. 2018. PMID: 30622658 Free PMC article. Review.
-
The role of Mycobacterium leprae phenolic glycolipid I (PGL-I) in serodiagnosis and in the pathogenesis of leprosy.Lepr Rev. 2011 Dec;82(4):344-57. Lepr Rev. 2011. PMID: 22439275 Review.
Cited by
-
Emerging applications of phage therapy and fecal virome transplantation for treatment of Clostridioides difficile infection: challenges and perspectives.Gut Pathog. 2023 May 9;15(1):21. doi: 10.1186/s13099-023-00550-3. Gut Pathog. 2023. PMID: 37161478 Free PMC article. Review.
-
Developing Recombinant Antibodies by Phage Display Against Infectious Diseases and Toxins for Diagnostics and Therapy.Front Cell Infect Microbiol. 2021 Jul 7;11:697876. doi: 10.3389/fcimb.2021.697876. eCollection 2021. Front Cell Infect Microbiol. 2021. PMID: 34307196 Free PMC article. Review.
-
HSP60 mimetic peptides from Mycobacterium leprae as new antigens for immunodiagnosis of Leprosy.AMB Express. 2023 Oct 27;13(1):120. doi: 10.1186/s13568-023-01625-9. AMB Express. 2023. PMID: 37891336 Free PMC article.
-
Bacilloscopy and polymerase chain reaction of slit-skin smears and anti-phenolic glycolipid-I serology for Hansen's disease diagnosis.Front Med (Lausanne). 2022 Aug 10;9:972244. doi: 10.3389/fmed.2022.972244. eCollection 2022. Front Med (Lausanne). 2022. PMID: 36035405 Free PMC article.
References
-
- Barbas C. F. (2001). Phage Display: a Laboratory Manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press.
-
- Bazan-Furini R., Motta A. C. F., Simão J. C. L., Tarquínio D. C., Marques W., Jr., Barbosa M. H., et al. (2011). Early detection of leprosy by examination of household contacts, determination of serum anti-PGL-I antibodies and consanguinity. Memórias do Instituto Oswaldo Cruz 106 536–540. 10.1590/s0074-02762011000500003 - DOI - PubMed
LinkOut - more resources
Full Text Sources

