Chronic Intra-Uterine Ureaplasma parvum Infection Induces Injury of the Enteric Nervous System in Ovine Fetuses
- PMID: 32256485
- PMCID: PMC7089942
- DOI: 10.3389/fimmu.2020.00189
Chronic Intra-Uterine Ureaplasma parvum Infection Induces Injury of the Enteric Nervous System in Ovine Fetuses
Erratum in
-
Corrigendum: Chronic Intra-Uterine Ureaplasma parvum Infection Induces Injury of the Enteric Nervous System in Ovine Fetuses.Front Immunol. 2020 Apr 15;11:672. doi: 10.3389/fimmu.2020.00672. eCollection 2020. Front Immunol. 2020. PMID: 32351513 Free PMC article.
Abstract
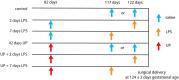
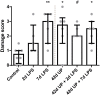
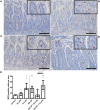
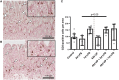
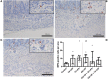
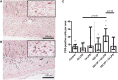
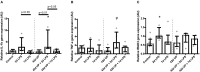
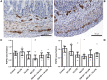
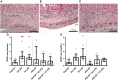
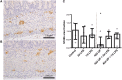
Background: Chorioamnionitis, inflammation of the fetal membranes during pregnancy, is often caused by intra-amniotic (IA) infection with single or multiple microbes. Chorioamnionitis can be either acute or chronic and is associated with adverse postnatal outcomes of the intestine, including necrotizing enterocolitis (NEC). Neonates with NEC have structural and functional damage to the intestinal mucosa and the enteric nervous system (ENS), with loss of enteric neurons and glial cells. Yet, the impact of acute, chronic, or repetitive antenatal inflammatory stimuli on the development of the intestinal mucosa and ENS has not been studied. The aim of this study was therefore to investigate the effect of acute, chronic, and repetitive microbial exposure on the intestinal mucosa, submucosa and ENS in premature lambs. Materials and Methods: A sheep model of pregnancy was used in which the ileal mucosa, submucosa, and ENS were assessed following IA exposure to lipopolysaccharide (LPS) for 2 or 7 days (acute), Ureaplasma parvum (UP) for 42 days (chronic), or repetitive microbial exposure (42 days UP with 2 or 7 days LPS). Results: IA LPS exposure for 7 days or IA UP exposure for 42 days caused intestinal injury and inflammation in the mucosal and submucosal layers of the gut. Repetitive microbial exposure did not further aggravate injury of the terminal ileum. Chronic IA UP exposure caused significant structural ENS alterations characterized by loss of PGP9.5 and S100β immunoreactivity, whereas these changes were not found after re-exposure of chronic UP-exposed fetuses to LPS for 2 or 7 days. Conclusion: The in utero loss of PGP9.5 and S100β immunoreactivity following chronic UP exposure corresponds with intestinal changes in neonates with NEC and may therefore form a novel mechanistic explanation for the association of chorioamnionitis and NEC.
Keywords: Ureaplasma parvum; chorioamnionitis; enteric nervous system; intra-amniotic infection; necrotizing enterocolitis; preterm birth; sheep.
Copyright © 2020 Heymans, de Lange, Hütten, Lenaerts, de Ruijter, Kessels, Rademakers, Melotte, Boesmans, Saito, Usuda, Stock, Spiller, Payne, Kramer, Newnham, Jobe, Kemp, van Gemert and Wolfs.
Figures
References
-
- Blencowe H, Cousens S, Oestergaard MZ, Chou D, Moller AB, Narwal R, et al. National, regional, and worldwide estimates of preterm birth rates in the year 2010 with time trends since 1990 for selected countries: a systematic analysis and implications. Lancet. (2012) 379:2162–72. 10.1016/S0140-6736(12)60820-4 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

