Human Intestinal Mononuclear Phagocytes in Health and Inflammatory Bowel Disease
- PMID: 32256490
- PMCID: PMC7093381
- DOI: 10.3389/fimmu.2020.00410
Human Intestinal Mononuclear Phagocytes in Health and Inflammatory Bowel Disease
Abstract
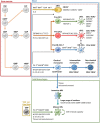
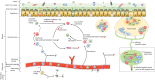
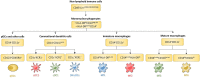
Inflammatory bowel disease (IBD), including Crohn's disease and ulcerative colitis, is a complex immune-mediated disease of the gastrointestinal tract that increases morbidity and negatively influences the quality of life. Intestinal mononuclear phagocytes (MNPs) have a crucial role in maintaining epithelial barrier integrity while controlling pathogen invasion by activating an appropriate immune response. However, in genetically predisposed individuals, uncontrolled immune activation to intestinal flora is thought to underlie the chronic mucosal inflammation that can ultimately result in IBD. Thus, MNPs are involved in fine-tuning mucosal immune system responsiveness and have a critical role in maintaining homeostasis or, potentially, the emergence of IBD. MNPs include monocytes, macrophages and dendritic cells, which are functionally diverse but highly complementary. Despite their crucial role in maintaining intestinal homeostasis, specific functions of human MNP subsets are poorly understood, especially during diseases such as IBD. Here we review the current understanding of MNP ontogeny, as well as the recently identified human intestinal MNP subsets, and discuss their role in health and IBD.
Keywords: crohn's disease; dendritic cells; intestine; macrophages; ulcerative colitis.
Copyright © 2020 Caër and Wick.
Figures



Similar articles
-
Subsets of mononuclear phagocytes are enriched in the inflamed colons of patients with IBD.BMC Immunol. 2019 Nov 12;20(1):42. doi: 10.1186/s12865-019-0322-z. BMC Immunol. 2019. PMID: 31718550 Free PMC article.
-
Diversity and functions of intestinal mononuclear phagocytes.Mucosal Immunol. 2017 Jul;10(4):845-864. doi: 10.1038/mi.2017.22. Epub 2017 Apr 5. Mucosal Immunol. 2017. PMID: 28378807 Review.
-
Development, validation and implementation of an in vitro model for the study of metabolic and immune function in normal and inflamed human colonic epithelium.Dan Med J. 2015 Jan;62(1):B4973. Dan Med J. 2015. PMID: 25557335 Review.
-
Group 3 ILCs: Peacekeepers or Troublemakers? What's Your Gut Telling You?!Front Immunol. 2019 Apr 5;10:676. doi: 10.3389/fimmu.2019.00676. eCollection 2019. Front Immunol. 2019. PMID: 31024537 Free PMC article. Review.
-
TREM-1+ Macrophages Define a Pathogenic Cell Subset in the Intestine of Crohn's Disease Patients.J Crohns Colitis. 2021 Aug 2;15(8):1346-1361. doi: 10.1093/ecco-jcc/jjab022. J Crohns Colitis. 2021. PMID: 33537747 Free PMC article.
Cited by
-
Effect of Curcumin Plus Piperine on Redox Imbalance, Fecal Calprotectin and Cytokine Levels in Inflammatory Bowel Disease Patients: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial.Pharmaceuticals (Basel). 2024 Jun 28;17(7):849. doi: 10.3390/ph17070849. Pharmaceuticals (Basel). 2024. PMID: 39065700 Free PMC article.
-
Interleukin-26 Expression in Inflammatory Bowel Disease and Its Immunoregulatory Effects on Macrophages.Front Med (Lausanne). 2022 Apr 6;9:797135. doi: 10.3389/fmed.2022.797135. eCollection 2022. Front Med (Lausanne). 2022. PMID: 35463017 Free PMC article.
-
Characterization of intestinal mononuclear phagocyte subsets in young ruminants at homeostasis and during Cryptosporidium parvum infection.Front Immunol. 2024 May 2;15:1379798. doi: 10.3389/fimmu.2024.1379798. eCollection 2024. Front Immunol. 2024. PMID: 38756777 Free PMC article.
-
Alteration of the Inflammatory and Anti-Inflammatory Cytokine Profiles of Peripheral Blood Mononuclear Cell in Crohn's Disease Patients after Following up.Iran J Public Health. 2022 Jul;51(7):1648-1657. doi: 10.18502/ijph.v51i7.10099. Iran J Public Health. 2022. PMID: 36248290 Free PMC article.
-
A Versatile Intestine-on-Chip System for Deciphering the Immunopathogenesis of Inflammatory Bowel Disease.Adv Healthc Mater. 2024 Mar;13(7):e2302454. doi: 10.1002/adhm.202302454. Epub 2024 Feb 11. Adv Healthc Mater. 2024. PMID: 38253407 Free PMC article.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

