A Versatile High Throughput Screening Platform for Plant Metabolic Engineering Highlights the Major Role of ABI3 in Lipid Metabolism Regulation
- PMID: 32256511
- PMCID: PMC7090168
- DOI: 10.3389/fpls.2020.00288
A Versatile High Throughput Screening Platform for Plant Metabolic Engineering Highlights the Major Role of ABI3 in Lipid Metabolism Regulation
Abstract
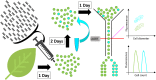
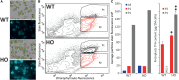
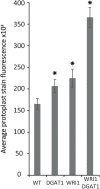
Traditional functional genetic studies in crops are time consuming, complicated and cannot be readily scaled up. The reason is that mutant or transformed crops need to be generated to study the effect of gene modifications on specific traits of interest. However, many crop species have a complex genome and a long generation time. As a result, it usually takes several months to over a year to obtain desired mutants or transgenic plants, which represents a significant bottleneck in the development of new crop varieties. To overcome this major issue, we are currently establishing a versatile plant genetic screening platform, amenable to high throughput screening in almost any crop species, with a unique workflow. This platform combines protoplast transformation and fluorescence activated cell sorting. Here we show that tobacco protoplasts can accumulate high levels of lipid if transiently transformed with genes involved in lipid biosynthesis and can be sorted based on lipid content. Hence, protoplasts can be used as a predictive tool for plant lipid engineering. Using this newly established strategy, we demonstrate the major role of ABI3 in plant lipid accumulation. We anticipate that this workflow can be applied to numerous highly valuable metabolic traits other than storage lipid accumulation. This new strategy represents a significant step toward screening complex genetic libraries, in a single experiment and in a matter of days, as opposed to years by conventional means.
Keywords: fluorescence activated cell sorting; high throughput screening; lipid accumulation; metabolic engineering; protoplast.
Copyright © 2020 Pouvreau, Blundell, Vohra, Zwart, Arndell, Singh and Vanhercke.
Figures

Similar articles
-
A Robotic Platform for High-throughput Protoplast Isolation and Transformation.J Vis Exp. 2016 Sep 27;(115):54300. doi: 10.3791/54300. J Vis Exp. 2016. PMID: 27768035 Free PMC article.
-
Enhancement of Plant Productivity in the Post-Genomics Era.Curr Genomics. 2016 Aug;17(4):295-6. doi: 10.2174/138920291704160607182507. Curr Genomics. 2016. PMID: 27499678 Free PMC article.
-
A selection strategy in plant transformation based on antisense oligodeoxynucleotide inhibition.Plant J. 2014 Mar;77(6):954-61. doi: 10.1111/tpj.12433. Epub 2014 Mar 8. Plant J. 2014. PMID: 24438514
-
Synthetic redesign of plant lipid metabolism.Plant J. 2016 Jul;87(1):76-86. doi: 10.1111/tpj.13172. Epub 2016 Jun 20. Plant J. 2016. PMID: 27483205 Free PMC article. Review.
-
Genetically modified (GM) crops: milestones and new advances in crop improvement.Theor Appl Genet. 2016 Sep;129(9):1639-55. doi: 10.1007/s00122-016-2747-6. Epub 2016 Jul 5. Theor Appl Genet. 2016. PMID: 27381849 Review.
Cited by
-
Non-targeted Lipidomics Using a Robust and Reproducible Lipid Separation Using UPLC with Charged Surface Hybrid Technology and High-Resolution Mass Spectrometry.Methods Mol Biol. 2022;2396:175-186. doi: 10.1007/978-1-0716-1822-6_13. Methods Mol Biol. 2022. PMID: 34786683
-
High oil accumulation in tuber of yellow nutsedge compared to purple nutsedge is associated with more abundant expression of genes involved in fatty acid synthesis and triacylglycerol storage.Biotechnol Biofuels. 2021 Mar 2;14(1):54. doi: 10.1186/s13068-021-01909-x. Biotechnol Biofuels. 2021. PMID: 33653389 Free PMC article.
-
Lipid Metabolism and Improvement in Oilseed Crops: Recent Advances in Multi-Omics Studies.Metabolites. 2023 Nov 23;13(12):1170. doi: 10.3390/metabo13121170. Metabolites. 2023. PMID: 38132852 Free PMC article. Review.
-
Natural drug delivery systems for the treatment of neurodegenerative diseases.Mol Biol Rep. 2025 Feb 10;52(1):217. doi: 10.1007/s11033-025-10286-9. Mol Biol Rep. 2025. PMID: 39928236 Review.
-
Nature's laboratory: plant metabolic engineering methods using phenylpropanoids as a case study.Biotechnol Biofuels Bioprod. 2025 Jul 24;18(1):81. doi: 10.1186/s13068-025-02684-9. Biotechnol Biofuels Bioprod. 2025. PMID: 40708042 Free PMC article. Review.
References
-
- Alameldin H., Izadi-Darbandi A., Smith S. A., Balan V., Jones A. D., Sticklen M. (2017). Production of seed-like storage lipids and increase in oil bodies in corn (Maize; Zea mays L.) vegetative biomass. Ind. Crops Products 108 526–534. 10.1016/j.indcrop.2017.07.021 - DOI
LinkOut - more resources
Full Text Sources
Research Materials

