Neuroprotection against Amyloid- β-Induced DNA Double-Strand Breaks Is Mediated by Multiple Retinoic Acid-Dependent Pathways
- PMID: 32256561
- PMCID: PMC7109576
- DOI: 10.1155/2020/9369815
Neuroprotection against Amyloid- β-Induced DNA Double-Strand Breaks Is Mediated by Multiple Retinoic Acid-Dependent Pathways
Abstract
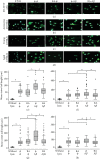
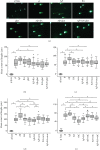
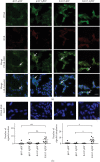
In this study, we have investigated the role of all-trans-retinoic acid (RA) as a neuroprotective agent against Aβ 1-42-induced DNA double-strand breaks (DSBs) in neuronal SH-SY5Y and astrocytic DI TNC1 cell lines and in murine brain tissues, by single-cell gel electrophoresis. We showed that RA does not only repair Aβ 1-42-induced DSBs, as already known, but also prevents their occurrence. This effect is independent of that of other antioxidants studied, such as vitamin C, and appears to be mediated, at least in part, by changes in expression, not of the RARα, but of the PPARβ/δ and of antiamyloidogenic proteins, such as ADAM10, implying a decreased production of endogenous Aβ. Whereas Aβ 1-42 needs transcription and translation for DSB production, RA protects against Aβ 1-42-induced DSBs at the posttranslational level through both the RARα/β/γ and PPARβ/δ receptors as demonstrated by using specific antagonists. Furthermore, it could be shown by a proximity ligation assay that the PPARβ/δ-RXR interactions, not the RARα/β/γ-RXR interactions, increased in the cells when a 10 min RA treatment was followed by a 20 min Aβ 1-42 treatment. Thus, the PPARβ/δ receptor, known for its antiapoptotic function, might for these short-time treatments play a role in neuroprotection via PPARβ/δ-RXR heterodimerization and possibly expression of antiamyloidogenic genes. Overall, this study shows that RA can not only repair Aβ 1-42-induced DSBs but also prevent them via the RARα/β/γ and PPARβ/δ receptors. It suggests that the RA-dependent pathways belong to an anti-DSB Adaptative Gene Expression (DSB-AGE) system that can be targeted by prevention strategies to preserve memory in Alzheimer's disease and aging.
Copyright © 2020 Julien Colas et al.
Conflict of interest statement
The authors declare no actual or potential conflict of interest.
Figures


Similar articles
-
Preferential Involvement of BRCA1/BARD1, Not Tip60/Fe65, in DNA Double-Strand Break Repair in Presenilin-1 P117L Alzheimer Models.Neural Plast. 2022 Feb 21;2022:3172861. doi: 10.1155/2022/3172861. eCollection 2022. Neural Plast. 2022. PMID: 35237315 Free PMC article.
-
The Vitamin A Derivative All-Trans Retinoic Acid Repairs Amyloid-β-Induced Double-Strand Breaks in Neural Cells and in the Murine Neocortex.Neural Plast. 2016;2016:3707406. doi: 10.1155/2016/3707406. Epub 2016 Jan 3. Neural Plast. 2016. PMID: 26881107 Free PMC article.
-
Piper sarmentosum Roxb. confers neuroprotection on beta-amyloid (Aβ)-induced microglia-mediated neuroinflammation and attenuates tau hyperphosphorylation in SH-SY5Y cells.J Ethnopharmacol. 2018 May 10;217:187-194. doi: 10.1016/j.jep.2018.02.025. Epub 2018 Feb 17. J Ethnopharmacol. 2018. PMID: 29462698
-
Peroxisome proliferator-activated receptor β/δ (PPARβ/δ) protects against ceramide-induced cellular toxicity in rat brain astrocytes and neurons by activation of ceramide kinase.Mol Cell Neurosci. 2014 Mar;59:127-34. doi: 10.1016/j.mcn.2014.01.008. Epub 2014 Feb 8. Mol Cell Neurosci. 2014. PMID: 24513118
-
Vitamin D3 protects against Aβ peptide cytotoxicity in differentiated human neuroblastoma SH- SY5Y cells: A role for S1P1/p38MAPK/ATF4 axis.Neuropharmacology. 2017 Apr;116:328-342. doi: 10.1016/j.neuropharm.2017.01.003. Epub 2017 Jan 7. Neuropharmacology. 2017. PMID: 28077289
Cited by
-
Identification and characterization of the Hfq bacterial amyloid region DNA interactions.BBA Adv. 2021 Oct 30;1:100029. doi: 10.1016/j.bbadva.2021.100029. eCollection 2021. BBA Adv. 2021. PMID: 37082015 Free PMC article.
-
Amyloid and Tau Induce Cell Death Independently of TSPO Polymerization and Density Changes.ACS Omega. 2021 Jul 14;6(29):18719-18727. doi: 10.1021/acsomega.1c01678. eCollection 2021 Jul 27. ACS Omega. 2021. PMID: 34337211 Free PMC article.
-
The Contribution of Hippocampal All-Trans Retinoic Acid (ATRA) Deficiency to Alzheimer's Disease: A Narrative Overview of ATRA-Dependent Gene Expression in Post-Mortem Hippocampal Tissue.Antioxidants (Basel). 2023 Oct 27;12(11):1921. doi: 10.3390/antiox12111921. Antioxidants (Basel). 2023. PMID: 38001775 Free PMC article. Review.
-
Preferential Involvement of BRCA1/BARD1, Not Tip60/Fe65, in DNA Double-Strand Break Repair in Presenilin-1 P117L Alzheimer Models.Neural Plast. 2022 Feb 21;2022:3172861. doi: 10.1155/2022/3172861. eCollection 2022. Neural Plast. 2022. PMID: 35237315 Free PMC article.
-
All-Trans Retinoic Acid Attenuates Blue Light-Induced Apoptosis of Retinal Photoreceptors by Upregulating MKP-1 Expression.Mol Neurobiol. 2021 Aug;58(8):4157-4168. doi: 10.1007/s12035-021-02380-3. Epub 2021 May 5. Mol Neurobiol. 2021. PMID: 33950345
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

