Two antibacterial and PPARα/γ-agonistic unsaturated keto fatty acids from a coral-associated actinomycete of the genus Micrococcus
- PMID: 32256847
- PMCID: PMC7082699
- DOI: 10.3762/bjoc.16.29
Two antibacterial and PPARα/γ-agonistic unsaturated keto fatty acids from a coral-associated actinomycete of the genus Micrococcus
Abstract
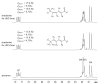
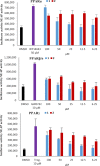
A pair of geometrically isomeric unsaturated keto fatty acids, (6E,8Z)- and (6E,8E)-5-oxo-6,8-tetradecadienoic acids (1 and 2), were isolated from the culture broth of an actinomycete of the genus Micrococcus, which was associated with a stony coral, Catalaphyllia sp. Their chemical structures were elucidated by spectroscopic analysis including NMR and MS, with special assistance of spin system simulation studies for the assignment of an E geometry at C8 in 2. As metabolites of microbes, compounds 1 and 2 are unprecedented in terms of bearing a 2,4-dienone system. Both 1 and 2 showed antibacterial activity against the plant pathogen Rhizobium radiobacter and the fish pathogen Tenacibaculum maritimum, with a contrasting preference that 1 is more effective to the former strain while 2 is so to the latter. In addition, compounds 1 and 2 displayed agonistic activity against peroxisome proliferator-activated receptors (PPARs) with an isoform specificity towards PPARα and PPARγ.
Keywords: Micrococcus; PPAR; antibacterial; coral; keto fatty acid.
Copyright © 2020, Sharma et al.; licensee Beilstein-Institut.
Figures
References
-
- Brinkmann C M, Marker A, Kurtböke D I. Diversity. 2017;9:No. 40. doi: 10.3390/d9040040. - DOI
LinkOut - more resources
Full Text Sources
Research Materials