Copper-promoted/copper-catalyzed trifluoromethylselenolation reactions
- PMID: 32256848
- PMCID: PMC7082709
- DOI: 10.3762/bjoc.16.30
Copper-promoted/copper-catalyzed trifluoromethylselenolation reactions
Abstract
Copper catalysis and, more generally, copper chemistry are pivotal for modern organofluorine chemistry. Major advances have been made in the field of trifluoromethylselenolations of organic compounds where copper catalysis played a crucial role. Recent developments in this field are highlighted in this minireview.
Keywords: copper catalysis; fluorine; homogenous catalysis; trifluoromethylselenolation.
Copyright © 2020, Ghiazza and Tlili; licensee Beilstein-Institut.
Figures


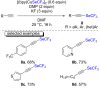
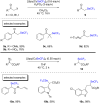
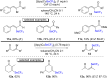

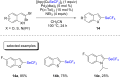



References
Publication types
LinkOut - more resources
Full Text Sources
