Architecture and synthesis of P , N-heterocyclic phosphine ligands
- PMID: 32256853
- PMCID: PMC7082614
- DOI: 10.3762/bjoc.16.35
Architecture and synthesis of P , N-heterocyclic phosphine ligands
Abstract
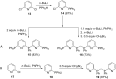
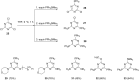
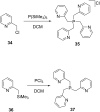
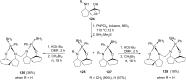
Diverse P,N-phosphine ligands reported to date have performed exceptionally well as auxiliary ligands in organometallic catalysis. Phosphines bearing 2-pyridyl moieties prominently feature in literature as compared to phosphines with five-membered N-heterocycles. This discussion seeks to paint a broad picture and consolidate different synthetic protocols and techniques for N-heterocyclic phosphine motifs. The introduction provides an account of P,N-phosphine ligands, and their structural and coordination benefits from combining heteroatoms with different basicity in one ligand. The body discusses the synthetic protocols which focus on P-C, P-N-bond formation, substrate and nucleophile types and different N-heterocycle construction strategies. Selected references are given in relation to the applications of the ligands.
Keywords: P,N-heterocyclic phosphines; ligand synthesis.
Copyright © 2020, Munzeiwa et al.; licensee Beilstein-Institut.
Figures





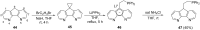







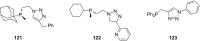






Similar articles
-
Multidentate 2-pyridyl-phosphine ligands - towards ligand tuning and chirality.Dalton Trans. 2017 Jan 17;46(3):814-824. doi: 10.1039/c6dt04390a. Dalton Trans. 2017. PMID: 28001157
-
Synthesis and photophysical studies of tetrazolate-based Eu(III) photoluminescent ternary complexes containing N-heterocyclic phosphine oxides auxiliary co-ligands.Luminescence. 2016 Aug;31(5):1085-90. doi: 10.1002/bio.3075. Epub 2015 Dec 17. Luminescence. 2016. PMID: 26679054
-
N-Heterocyclic silylenes as ambiphilic activators and ligands.Dalton Trans. 2021 May 25;50(20):6752-6765. doi: 10.1039/d1dt00617g. Dalton Trans. 2021. PMID: 34032246
-
Nucleophilic phosphine organocatalysis: a practical synthetic strategy for the drug-like nitrogen heterocyclic framework construction.Mini Rev Med Chem. 2013 May 1;13(6):836-44. doi: 10.2174/1389557511313060006. Mini Rev Med Chem. 2013. PMID: 23544464 Review.
-
Modular phospholane ligands in asymmetric catalysis.Acc Chem Res. 2000 Jun;33(6):363-72. doi: 10.1021/ar990085c. Acc Chem Res. 2000. PMID: 10891054 Review.
Cited by
-
Coinage Metal Complexes of Bis(quinoline-2-ylmethyl)phenylphosphine-Simple Reactions Can Lead to Unprecedented Results.ChemistryOpen. 2022 Mar;11(3):e202100224. doi: 10.1002/open.202100224. Epub 2022 Feb 10. ChemistryOpen. 2022. PMID: 35146971 Free PMC article.
-
The Backbone of Success of P,N-Hybrid Ligands: Some Recent Developments.Molecules. 2022 Sep 23;27(19):6293. doi: 10.3390/molecules27196293. Molecules. 2022. PMID: 36234830 Free PMC article. Review.
-
Enantioselective copper-catalyzed hydrophosphination of alkenyl isoquinolines.Chem Sci. 2023 Mar 21;14(16):4413-4417. doi: 10.1039/d2sc06950d. eCollection 2023 Apr 26. Chem Sci. 2023. PMID: 37123192 Free PMC article.
-
Recent Progress in Cyclic Aryliodonium Chemistry: Syntheses and Applications.Chem Rev. 2023 Jan 17;123(4):1364-416. doi: 10.1021/acs.chemrev.2c00591. Online ahead of print. Chem Rev. 2023. PMID: 36649301 Free PMC article. Review.
References
-
- Debono N, Canac Y, Duhayon C, Chauvin R. Eur J Inorg Chem. 2008;(19):2991–2999. doi: 10.1002/ejic.200800157. - DOI
-
- Benoit W L, Parvez M, Keay B A. Tetrahedron: Asymmetry. 2009;20:69–77. doi: 10.1016/j.tetasy.2008.12.027. - DOI
-
- Berners-Price S J, Bowen R J, Galettis P, Healy P C, McKeage M J. Coord Chem Rev. 1999;185–186:823–836. doi: 10.1016/s0010-8545(99)00039-9. - DOI
Publication types
LinkOut - more resources
Full Text Sources
