2019 APHRS expert consensus statement on three-dimensional mapping systems for tachycardia developed in collaboration with HRS, EHRA, and LAHRS
- PMID: 32256872
- PMCID: PMC7132207
- DOI: 10.1002/joa3.12308
2019 APHRS expert consensus statement on three-dimensional mapping systems for tachycardia developed in collaboration with HRS, EHRA, and LAHRS
Figures
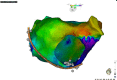
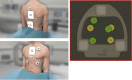
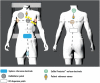



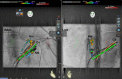
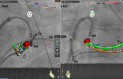


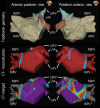
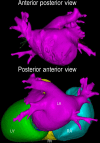


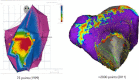
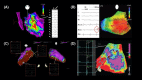
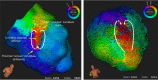
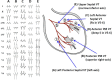

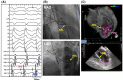



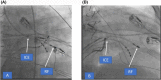

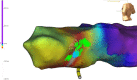

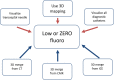
References
-
- Asirvatham S, Narayan O. Advanced catheter mapping and navigation system In: Huang S, Wood M, editors. Catheter ablation of cardiac arrhythmias. Philadelphia, PA: Saunders/Elsevier, 2006; p. 135–61.
-
- Gurevitz OT, Glikson M, Asirvatham S, Kester TA, Grice SK, Munger TM, et al. Use of advanced mapping systems to guide ablation in complex cases: experience with noncontact mapping and electroanatomic mapping systems. Pacing Clin Electrophysiol. 2005;28:316–23. - PubMed
-
- Corrado D, Basso C, Leoni L, Tokajuk B, Bauce B, Frigo G, et al. Three‐dimensional electroanatomic voltage mapping increases accuracy of diagnosing arrhythmogenic right ventricular cardiomyopathy/dysplasia. Circulation. 2005;111:3042–50. - PubMed
-
- Nakagawa H, Shah N, Matsudaira K, Overholt E, Chandrasekaran K, Beckman KJ, et al. Characterization of reentrant circuit in macroreentrant right atrial tachycardia after surgical repair of congenital heart disease: isolated channels between scars allow "focal" ablation. Circulation. 2001;103:699–709. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

