Enzymes involved in folate metabolism and its implication for cancer treatment
- PMID: 32256983
- PMCID: PMC7075736
- DOI: 10.4162/nrp.2020.14.2.95
Enzymes involved in folate metabolism and its implication for cancer treatment
Abstract
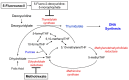
Background/objectives: Folate plays a critical role in DNA synthesis and methylation. Intracellular folate homeostasis is maintained by the enzymes folylpolyglutamate synthase (FPGS) and γ-glutamyl hydrolase (GGH). FPGS adds glutamate residues to folate upon its entry into the cell through a process known as polyglutamylation to enhance folate retention in the cell and to maintain a steady supply of utilizable folate derivatives for folate-dependent enzyme reactions. Thereafter, GGH catalyzes the hydrolysis of polyglutamylated folate into monoglutamylated folate, which can subsequently be exported from the cell. The objective of this review is to summarize the scientific evidence available on the effects of intracellular folate homeostasis-associated enzymes on cancer chemotherapy.
Methods: This review discusses the effects of FPGS and GGH on chemosensitivity to cancer chemotherapeutic agents such as antifolates, such as methotrexate, and 5-fluorouracil.
Results and discussion: Polyglutamylated (anti)folates are better substrates for intracellular folate-dependent enzymes and retained for longer within cells. In addition to polyglutamylation of (anti)folates, FPGS and GGH modulate intracellular folate concentrations, which are an important determinant of chemosensitivity of cancer cells toward chemotherapeutic agents. Therefore, FPGS and GGH affect chemosensitivity to antifolates and 5-fluorouracil by altering intracellular retention status of antifolates and folate cofactors such as 5,10-methylenetetrahydrofolate, subsequently influencing the cytotoxic effects of 5-fluorouracil, respectively. Generally, high FPGS and/or low GGH activity is associated with increased chemosensitivity of cancer cells to methotrexate and 5-fluorouracil, while low FPGS and/or high GGH activity seems to correspond to resistance to these drugs. Further preclinical and clinical studies elucidating the pharmocogenetic ramifications of these enzyme-induced changes are warranted to provide a framework for developing rational, effective, safe, and customized chemotherapeutic practices.
Keywords: 5-fluorouracil; Folate; antifolates; cancer; chemotherapy.
©2020 The Korean Nutrition Society and the Korean Society of Community Nutrition.
Conflict of interest statement
CONFLICT OF INTEREST: The author declares no potential conflicts of interests.
Figures
References
-
- Shane B. Folate chemistry and metabolism. In: Bailey LB, editor. Folate in Health and Disease. Boca Raton (FL): CRC Press; 2010. pp. 1–24.
-
- Gropper SAS, Smith JL, Groff JL. Advanced Nutrition and Human Metabolism. Belmont (CA): Wadsworth/Cengage Learning; 2009.
-
- Kim YI. Role of folate in colon cancer development and progression. J Nutr. 2003;133:3731S–3739S. - PubMed
-
- Kim YI. Folate and carcinogenesis: evidence, mechanisms, and implications. J Nutr Biochem. 1999;10:66–88. - PubMed
-
- Kim YI. Folate and colorectal cancer: an evidence-based critical review. Mol Nutr Food Res. 2007;51:267–292. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous