Crystal structure of the C-terminal domain of DENR
- PMID: 32257053
- PMCID: PMC7114459
- DOI: 10.1016/j.csbj.2020.03.009
Crystal structure of the C-terminal domain of DENR
Abstract
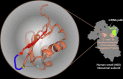
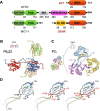
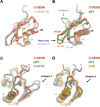
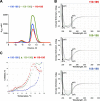
The density regulated protein (DENR) forms a stable heterodimer with malignant T-cell-amplified sequence 1 (MCT-1). DENR-MCT-1 heterodimer then participates in regulation of non-canonical translation initiation and ribosomal recycling. The N-terminal domain of DENR interacts with MCT-1 and carries a classical tetrahedral zinc ion-binding site, which is crucial for the dimerization. DENR-MCT-1 binds the small (40S) ribosomal subunit through interactions between MCT-1 and helix h24 of the 18S rRNA, and through interactions between the C-terminal domain of DENR and helix h44 of the 18S rRNA. This later interaction occurs in the vicinity of the P site that is also the binding site for canonical translation initiation factor eIF1, which plays the key role in initiation codon selection and scanning. Sequence homology modeling and a low-resolution crystal structure of the DENR-MCT-1 complex with the human 40S subunit suggests that the C-terminal domain of DENR and eIF1 adopt a similar fold. Here we present the crystal structure of the C-terminal domain of DENR determined at 1.74 Å resolution, which confirms its resemblance to eIF1 and advances our understanding of the mechanism by which DENR-MCT-1 regulates non-canonical translation initiation and ribosomal recycling.
Keywords: Density regulated protein (DENR); Protein synthesis regulation; Translation initiation; Translation recycling; Translation reinitiation.
© 2020 The Authors.
Figures



References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
