Control of synchronization ratios in clock/cell cycle coupling by growth factors and glucocorticoids
- PMID: 32257354
- PMCID: PMC7062057
- DOI: 10.1098/rsos.192054
Control of synchronization ratios in clock/cell cycle coupling by growth factors and glucocorticoids
Abstract
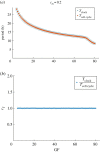
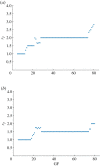
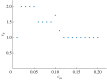
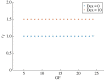
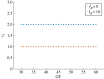
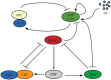
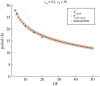
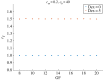
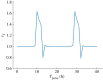
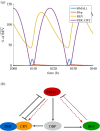
The cell cycle and the circadian clock are essential cyclic cellular processes often synchronous in healthy cells. In this work, we use previously developed mathematical models of the mammalian cell cycle and circadian cellular clock in order to investigate their dynamical interactions. Firstly, we study unidirectional cell cycle → clock coupling by proposing a mechanism of mitosis promoting factor (MPF)-controlled REV-ERBα degradation. Secondly, we analyse a bidirectional coupling configuration, where we add the CLOCK : BMAL1-mediated MPF repression via the WEE1 kinase to the first system. Our simulations reproduce ratios of clock to cell cycle period in agreement with experimental observations and give predictions of the system's synchronization state response to a variety of control parameters. Specifically, growth factors accelerate the coupled oscillators and dexamethasone (Dex) drives the system from a 1 : 1 to a 3 : 2 synchronization state. Furthermore, simulations of a Dex pulse reveal that certain time regions of pulse application drive the system from 1 : 1 to 3 : 2 synchronization while others have no effect, revealing the existence of a responsive and an irresponsive system's phase, a result we contextualize with observations on the segregation of Dex-treated cells into two populations.
Keywords: cell cycle; circadian clock; control parameters; coupled oscillators; modelling and simulation; synchronization ratios.
© 2020 The Authors.
Conflict of interest statement
We declare we have no competing interests.
Figures


References
Associated data
LinkOut - more resources
Full Text Sources
Miscellaneous
