Double negative T cells, a potential biomarker for systemic lupus erythematosus
- PMID: 32257532
- PMCID: PMC7093895
- DOI: 10.1093/pcmedi/pbaa001
Double negative T cells, a potential biomarker for systemic lupus erythematosus
Abstract
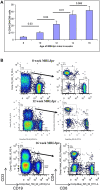
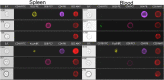
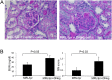
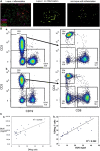
Systemic lupus erythematosus (SLE) is an autoimmune disease that is a challenge to diagnose and treat. There is an urgent need for biomarkers to help define organ involvement, and more effective therapies. A unique population of T cells, the CD3+CD4-CD8- (DNeg) cells, is significantly increased in lupus patients. Twenty-seven cases (53%) of pediatric SLE patients had elevated DNeg cells in their peripheral blood, which correlated with kidney function (R2 = 0.54). Significant infiltration of DNeg cells was observed in both adult and pediatric lupus kidneys by immunofluorescence. For the first time, this study provides direct evidence that DNeg cells facilitate kidney injury in preclinical 8-week-old MRL/lpr lupus mice. In lupus mice, the increase in DNeg cells tracked with worsening disease and correlated with kidney function (R2 = 0.85). Our results show that DNeg cells per se can cause kidney dysfunction, increase in number with increase in disease pathology, and could serve as a potential biomarker.
Keywords: CD3+CD4−CD8− T cells; glomerulonephritis; inflammation; lupus.
© The Author(s) 2020. Published by Oxford University Press on behalf of West China School of Medicine & West China Hospital of Sichuan University.
Figures

References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
