Transplantation of ACE2- Mesenchymal Stem Cells Improves the Outcome of Patients with COVID-19 Pneumonia
- PMID: 32257537
- PMCID: PMC7069465
- DOI: 10.14336/AD.2020.0228
Transplantation of ACE2- Mesenchymal Stem Cells Improves the Outcome of Patients with COVID-19 Pneumonia
Abstract
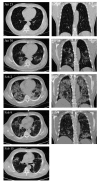
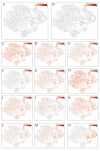
A coronavirus (HCoV-19) has caused the novel coronavirus disease (COVID-19) outbreak in Wuhan, China. Preventing and reversing the cytokine storm may be the key to save the patients with severe COVID-19 pneumonia. Mesenchymal stem cells (MSCs) have been shown to possess a comprehensive powerful immunomodulatory function. This study aims to investigate whether MSC transplantation improves the outcome of 7 enrolled patients with COVID-19 pneumonia in Beijing YouAn Hospital, China, from Jan 23, 2020 to Feb 16, 2020. The clinical outcomes, as well as changes of inflammatory and immune function levels and adverse effects of 7 enrolled patients were assessed for 14 days after MSC injection. MSCs could cure or significantly improve the functional outcomes of seven patients without observed adverse effects. The pulmonary function and symptoms of these seven patients were significantly improved in 2 days after MSC transplantation. Among them, two common and one severe patient were recovered and discharged in 10 days after treatment. After treatment, the peripheral lymphocytes were increased, the C-reactive protein decreased, and the overactivated cytokine-secreting immune cells CXCR3+CD4+ T cells, CXCR3+CD8+ T cells, and CXCR3+ NK cells disappeared in 3-6 days. In addition, a group of CD14+CD11c+CD11bmid regulatory DC cell population dramatically increased. Meanwhile, the level of TNF-α was significantly decreased, while IL-10 increased in MSC treatment group compared to the placebo control group. Furthermore, the gene expression profile showed MSCs were ACE2- and TMPRSS2- which indicated MSCs are free from COVID-19 infection. Thus, the intravenous transplantation of MSCs was safe and effective for treatment in patients with COVID-19 pneumonia, especially for the patients in critically severe condition.
Keywords: ACE2 negative; COVID-19; cell transplantation; function recovery; immunomodulation; mesenchymal stem cells.
Copyright: © 2020 Leng et al.
Conflict of interest statement
Conflicts of interest We have no conflicts of interest.
Figures



Comment in
-
Adipose-derived stromal stem cells (ASCs) as a new regenerative immediate therapy combating coronavirus (COVID-19)-induced pneumonia.Expert Opin Biol Ther. 2020 Jul;20(7):711-716. doi: 10.1080/14712598.2020.1761322. Epub 2020 Apr 29. Expert Opin Biol Ther. 2020. PMID: 32329380 Free PMC article. No abstract available.
-
Epidermal Neural Crest Stem Cells as a Perspective for COVID-19 Treatment.Stem Cell Rev Rep. 2021 Feb;17(1):291-292. doi: 10.1007/s12015-020-10028-3. Stem Cell Rev Rep. 2021. PMID: 32815107 Free PMC article. No abstract available.
References
-
- Munster VJ, Koopmans M, van Doremalen N, van Riel D, de Wit E (2020). A Novel Coronavirus Emerging in China — Key Questions for Impact Assessment. New England Journal of Medicine. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
