Hypoxia-Induced Degenerative Protein Modifications Associated with Aging and Age-Associated Disorders
- PMID: 32257546
- PMCID: PMC7069466
- DOI: 10.14336/AD.2019.0604
Hypoxia-Induced Degenerative Protein Modifications Associated with Aging and Age-Associated Disorders
Abstract
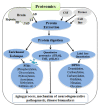
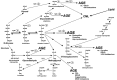
Aging is an inevitable time-dependent decline of various physiological functions that finally leads to death. Progressive protein damage and aggregation have been proposed as the root cause of imbalance in regulatory processes and risk factors for aging and neurodegenerative diseases. Oxygen is a modulator of aging. The oxygen-deprived conditions (hypoxia) leads to oxidative stress, cellular damage and protein modifications. Despite unambiguous evidence of the critical role of spontaneous non-enzymatic Degenerative Protein Modifications (DPMs) such as oxidation, glycation, carbonylation, carbamylation, and deamidation, that impart deleterious structural and functional protein alterations during aging and age-associated disorders, the mechanism that mediates these modifications is poorly understood. This review summarizes up-to-date information and recent developments that correlate DPMs, aging, hypoxia, and age-associated neurodegenerative diseases. Despite numerous advances in the study of the molecular hallmark of aging, hypoxia, and degenerative protein modifications during aging and age-associated pathologies, a major challenge remains there to dissect the relative contribution of different DPMs in aging (either natural or hypoxia-induced) and age-associated neurodegeneration.
Keywords: aging; dementia; hypoxia; neurodegenerative disease; protein aggregation; proteomics; proteostasis.
Copyright: © 2020 Adav et al.
Conflict of interest statement
Conflicts of interest The authors declare no conflict of interest.
Figures


References
-
- Viña J, Borrás C, Miquel J (2007). Theories of ageing. IUBMB life, 59:249-254. - PubMed
-
- Cornelius E (1972). Increased incidence of lymphomas in thymectomized mice--evidence for an immunological theory of aging. Experientia, 28:459. - PubMed
-
- Harman D (1956). Aging: a theory based on free radical and radiation chemistry. J Gerontol, 11:298-300. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
