Highly Stereoselective Synthesis of Fused Cyclopropane-γ-Lactams via Biocatalytic Iron-Catalyzed Intramolecular Cyclopropanation
- PMID: 32257580
- PMCID: PMC7111458
- DOI: 10.1021/acscatal.9b05383
Highly Stereoselective Synthesis of Fused Cyclopropane-γ-Lactams via Biocatalytic Iron-Catalyzed Intramolecular Cyclopropanation
Abstract
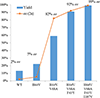
We report the development of an iron-based biocatalytic strategy for the asymmetric synthesis of fused cyclopropane-γ-lactams, which are key structural motifs found in synthetic drugs and bioactive natural products. Using a combination of mutational landscape and iterative site-saturation mutagenesis, sperm whale myoglobin was evolved into a biocatalyst capable of promoting the cyclization of a diverse range of allyl diazoacetamide substrates into the corresponding bicyclic lactams in high yields and with high enantioselectivity (up to 99% ee). These biocatalytic transformations can be performed in whole cells and could be leveraged to enable the efficient (chemo)enzymatic construction of chiral cyclopropane-γ-lactams as well as β-cyclopropyl amines and cyclopropane-fused pyrrolidines, as valuable building blocks and synthons for medicinal chemistry and natural product synthesis.
Keywords: Myoglobin; carbene transfer; cyclopropanation; lactams; protein engineering.
Conflict of interest statement
Notes The authors declare no competing financial interest.
Figures
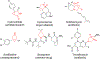


Similar articles
-
An Enzymatic Platform for the Highly Enantioselective and Stereodivergent Construction of Cyclopropyl-δ-lactones.Angew Chem Int Ed Engl. 2020 Nov 23;59(48):21634-21639. doi: 10.1002/anie.202007953. Epub 2020 Sep 17. Angew Chem Int Ed Engl. 2020. PMID: 32667122 Free PMC article.
-
Stereodivergent Intramolecular Cyclopropanation Enabled by Engineered Carbene Transferases.J Am Chem Soc. 2019 Jun 12;141(23):9145-9150. doi: 10.1021/jacs.9b02700. Epub 2019 May 29. J Am Chem Soc. 2019. PMID: 31099569 Free PMC article.
-
A Diverse Library of Chiral Cyclopropane Scaffolds via Chemoenzymatic Assembly and Diversification of Cyclopropyl Ketones.J Am Chem Soc. 2021 Feb 10;143(5):2221-2231. doi: 10.1021/jacs.0c09504. Epub 2021 Jan 26. J Am Chem Soc. 2021. PMID: 33497207 Free PMC article.
-
[Construction of All-cis-substituted Cyclopropane and Total Synthesis of Avenaol].Yakugaku Zasshi. 2019;139(10):1259-1265. doi: 10.1248/yakushi.19-00130. Yakugaku Zasshi. 2019. PMID: 31582609 Review. Japanese.
-
Research on transition metals for the multicomponent synthesis of benzo-fused γ-lactams.RSC Adv. 2025 Jan 23;15(4):2334-2346. doi: 10.1039/d4ra08798d. eCollection 2025 Jan 23. RSC Adv. 2025. PMID: 39867320 Free PMC article. Review.
Cited by
-
Engineered and Artificial Metalloenzymes for Selective C-H Functionalization.Curr Opin Green Sustain Chem. 2021 Oct;31:100494. doi: 10.1016/j.cogsc.2021.100494. Epub 2021 Apr 8. Curr Opin Green Sustain Chem. 2021. PMID: 34395950 Free PMC article.
-
Stereoselective Construction of β-, γ-, and δ-Lactam Rings via Enzymatic C-H Amidation.Nat Catal. 2024 Jan;7(1):65-76. doi: 10.1038/s41929-023-01068-2. Epub 2023 Dec 6. Nat Catal. 2024. PMID: 38584987 Free PMC article.
-
Is it time for biocatalysis in fragment-based drug discovery?Chem Sci. 2020 Oct 7;11(41):11104-11112. doi: 10.1039/d0sc04103c. Chem Sci. 2020. PMID: 34094353 Free PMC article. Review.
-
Nature's Machinery, Repurposed: Expanding the Repertoire of Iron-Dependent Oxygenases.ACS Catal. 2020 Oct 16;10(20):12239-12255. doi: 10.1021/acscatal.0c03606. Epub 2020 Sep 28. ACS Catal. 2020. PMID: 33282461 Free PMC article.
-
An Enzymatic Platform for the Highly Enantioselective and Stereodivergent Construction of Cyclopropyl-δ-lactones.Angew Chem Int Ed Engl. 2020 Nov 23;59(48):21634-21639. doi: 10.1002/anie.202007953. Epub 2020 Sep 17. Angew Chem Int Ed Engl. 2020. PMID: 32667122 Free PMC article.
References
-
- Epstein JW, Brabander HJ, Fanshawe WJ, Hofmann CM, McKenzie TC, Safir SR, Osterberg AC, Cosulich DB, and Lovell FM 1-Aryl-3-azabicyclo[3.1.0]hexanes, a new series of nonnarcotic analgesic agents, J. Med. Chem. 1981, 24, 481–490. - PubMed
-
- Bacon BR, Gordon SC, Lawitz E, Marcellin P, Vierling JM, Zeuzem S, Poordad F, Goodman ZD, Sings HL, Boparai N, Burroughs M, Brass CA, Albrecht JK, Esteban R, and Investigators HR −. Boceprevir for previously treated chronic HCV genotype 1 infection, N. Engl. J. Med. 2011, 364, 1207–1217. - PMC - PubMed
-
- Micheli F, Cavanni P, Andreotti D, Arban R, Benedetti R, Bertani B, Bettati M, Bettelini L, Bonanomi G, Braggio S, Carletti R, Checchia A, Corsi M, Fazzolari E, Fontana S, Marchioro C, Merlo-Pich E, Negri M, Oliosi B, Ratti E, Read KD, Roscic M, Sartori I, Spada S, Tedesco G, Tarsi L, Terreni S, Visentini F, Zocchi A, Zonzini L, and Di Fabio R 6-(3,4-dichlorophenyl)-1-[(methyloxy)methyl]-3-azabicyclo[4.1.0]heptane: a new potent and selective triple reuptake inhibitor, J. Med. Chem. 2010, 53, 4989–5001. - PubMed
-
- Gootz TD, Zaniewski R, Haskell S, Schmieder B, Tankovic J, Girard D, Courvalin P, and Polzer RJ Activity of the new fluoroquinolone trovafloxacin (CP-99,219) against DNA gyrase and topoisomerase IV mutants of Streptococcus pneumoniae selected in vitro, Antimicrob. Agents Chemother. 1996, 40, 2691–2697. - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
