Stereoselective Cyclopropanation of Electron-Deficient Olefins with a Cofactor Redesigned Carbene Transferase Featuring Radical Reactivity
- PMID: 32257582
- PMCID: PMC7111255
- DOI: 10.1021/acscatal.9b02272
Stereoselective Cyclopropanation of Electron-Deficient Olefins with a Cofactor Redesigned Carbene Transferase Featuring Radical Reactivity
Abstract
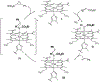
Engineered myoglobins and other hemoproteins have recently emerged as promising catalysts for asymmetric olefin cyclopropanation reactions via carbene transfer chemistry. Despite this progress, the transformation of electron-poor alkenes has proven very challenging using these systems. Here, we describe the design of a myoglobin-based carbene transferase incorporating a non-native iron-porphyrin cofactor and axial ligand, as an efficient catalyst for the asymmetric cyclopropanation of electron-deficient alkenes. Using this metalloenzyme, a broad range of both electron-rich and electron-deficient alkenes are cyclopropanated with high efficiency and high diastereo- and enantioselectivity (up to >99% de and ee). Mechanistic studies revealed that the expanded reaction scope of this carbene transferase is dependent upon the acquisition of metallocarbene radical reactivity as a result of the reconfigured coordination environment around the metal center. The radical-based reactivity of this system diverges from the electrophilic reactivity of myoglobin and most of known organometallic carbene transfer catalysts. This work showcases the value of cofactor redesign toward tuning and expanding the reactivity of metalloproteins in abiological reactions and it provides a biocatalytic solution to the asymmetric cyclopropanation of electrodeficient alkenes. The metallocarbene radical reactivity exhibited by this biocatalyst is anticipated to prove useful in the context of a variety of other synthetic transformations.
Keywords: Cyclopropanation; Hammett; carbene transfer catalysis; electrondeficient olefins; myoglobin; radical mechanism.
Figures
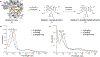





References
-
- Doyle MP, and Forbes DC Recent Advances in Asymmetric Catalytic Metal Carbene Transformations, Chem. Rev 1998, 98, 911–936. - PubMed
-
- Lebel H, Marcoux JF, Molinaro C, and Charette AB Stereoselective cyclopropanation reactions, Chem. Rev 2003, 103, 977–1050. - PubMed
-
- Davies HML, and Hedley SJ Intermolecular reactions of electron-rich heterocycles with copper and rhodium carbenoids, Chem. Soc. Rev 2007, 36, 1109–1119. - PubMed
-
- Pellissier H Recent developments in asymmetric cyclopropanation, Tetrahedron 2008, 64, 7041–7095.
Grants and funding
LinkOut - more resources
Full Text Sources
