Crystal Structure of the Ergothioneine Sulfoxide Synthase from Candidatus Chloracidobacterium thermophilum and Structure-Guided Engineering To Modulate Its Substrate Selectivity
- PMID: 32257583
- PMCID: PMC7133768
- DOI: 10.1021/acscatal.9b02054
Crystal Structure of the Ergothioneine Sulfoxide Synthase from Candidatus Chloracidobacterium thermophilum and Structure-Guided Engineering To Modulate Its Substrate Selectivity
Abstract
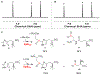
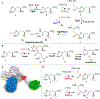
Ergothioneine is a thiohistidine derivative with potential benefits on many aging-related diseases. The central step of aerobic ergothioneine biosynthesis is the oxidative C-S bond formation reaction catalyzed by mononuclear nonheme iron sulfoxide synthases (EgtB and Egt1). Thus far, only the Mycobacterium thermoresistibile EgtB (EgtB Mth ) crystal structure is available, while the structural information for the more industrially attractive Egt1 enzyme is not. Herein, we reported the crystal structure of the ergothioneine sulfoxide synthase (EgtB Cth ) from Candidatus Chloracidobacterium thermophilum. EgtB Cth has both EgtB- and Egt1-type of activities. Guided by the structural information, we conducted Rosetta Enzyme Design calculations, and we biochemically demonstrated that EgtB Cth can be engineered more toward Egt1-type of activity. This study provides information regarding the factors governing the substrate selectivity in Egt1- and EgtB-catalysis and lays the groundwork for future sulfoxide synthase engineering toward the development of an effective ergothioneine process through a synthetic biology approach.
Keywords: Rosetta Enzyme Design; enzyme engineering; ergothioneine; nonheme iron enzyme; sulfur-containing natural product.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
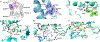
References
-
- Melville DB; Horner WH; Lubschez R Tissue Ergothioneine. J. Biol. Chem 1954, 206, 221–228. - PubMed
-
- Shires TK; Brummel MC; Pulido JS; Stegink LD Ergothioneine Distribution in Bovine and Porcine Ocular Tissues. Comp. Biochem. Physiol., Part C: Pharmacol., Toxicol. Endocrinol 1997, 117, 117–120. - PubMed
-
- Leone E; Mann T Ergothioneine in the Seminal Vesicle Secretion. Nature 1951, 168, 205–206. - PubMed
-
- Hartman PE Ergothioneine as Antioxidant. Methods Enzymol. 1990, 186, 310–318. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
