Functional Systems Derived from Nucleobase Self-assembly
- PMID: 32257750
- PMCID: PMC7110180
- DOI: 10.1002/open.201900363
Functional Systems Derived from Nucleobase Self-assembly
Abstract
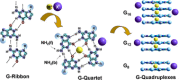
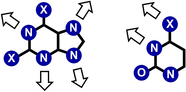
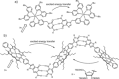
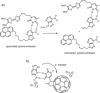
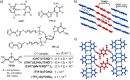
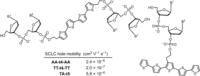
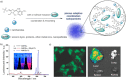
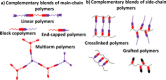
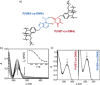
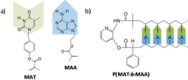
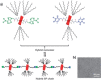
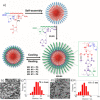
Dynamic and reversible non-covalent interactions endow synthetic systems and materials with smart adaptive functions that allow them to response to diverse stimuli, interact with external agents, or repair structural defects. Inspired by the outstanding performance and selectivity of DNA in living systems, scientists are increasingly employing Watson-Crick nucleobase pairing to control the structure and properties of self-assembled materials. Two sets of complementary purine-pyrimidine pairs (guanine:cytosine and adenine:thymine(uracil)) are available that provide selective and directional H-bonding interactions, present multiple metal-coordination sites, and exhibit rich redox chemistry. In this review, we highlight several recent examples that profit from these features and employ nucleobase interactions in functional systems and materials, covering the fields of energy/electron transfer, charge transport, adaptive nanoparticles, porous materials, macromolecule self-assembly, or polymeric materials with adhesive or self-healing ability.
Keywords: functional materials; hydrogen bonding; nucleobases; self-assembly; supramolecular chemistry.
© 2020 The Authors. Published by Wiley-VCH Verlag GmbH & Co. KGaA.
Conflict of interest statement
The authors declare no conflict of interest.
Figures












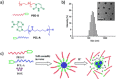


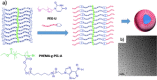

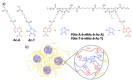

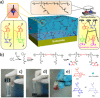

References
-
- None
-
- Steiner T., Angew. Chem. Int. Ed. 2002, 41, 48–76; - PubMed
- Angew. Chem. 2002, 114, 50–80;
-
- Prins L. J., Reinhoudt D. N., Timmerman P., Angew. Chem. Int. Ed. 2001, 40, 2382–2426; - PubMed
- Angew. Chem. 2001, 113, 2446–2492;
-
- Li Z.-T., Wu L.-Z., Hydrogen bonded supramolecular structures, Springer, Berlin, 2015;
-
- Gonzalez-Rodriguez D., Schenning A. P. H. J., Chem. Mater. 2011, 23, 310–325.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

