Methemoglobin determination by multi-component analysis in coho salmon (Oncorhynchus kisutch) possessing unstable hemoglobin
- PMID: 32257839
- PMCID: PMC7115134
- DOI: 10.1016/j.mex.2020.100836
Methemoglobin determination by multi-component analysis in coho salmon (Oncorhynchus kisutch) possessing unstable hemoglobin
Abstract
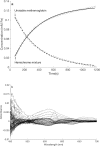
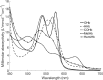
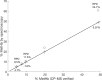
Hemoglobin derivatives are often quantified in blood to establish cardio-respiratory status and possible causes of impaired oxygen transport. The derivative known as methemoglobin results from oxidation of hemoglobin and is pathologically relevant because it cannot transport oxygen. In species and individuals possessing unstable methemoglobin, methemoglobin formation leads to rapid hemichrome formation and precipitation. Oxidizing reagents in standard methemoglobin analysis techniques therefore prevent accurate quantification of hemoglobin oxidative degradation products in species possessing unstable hemoglobin. In this study, we demonstrated that individual coho salmon (Oncorhynchus kisutch) possess unstable methemoglobin. Because molar absorptivities of coho methemoglobin, hemichrome and carboxyhemoglobin were significantly different from humans, the use of previous standard methods leads to an overestimation of methemoglobin in coho. Spontaneous conversion of methemoglobin to hemichrome was also demonstrated in Chinook (O. tshawytscha), pink (O. gorbuscha) and chum salmon (O. keta), but not steelhead (O. mykiss), indicating there may be a frequent need to account for unstable hemoglobin when quantifying methemoglobin in salmonids.•Our method builds upon multi-component analysis (MCA) by using a multivariate modeling technique to derive the coho-specific molar absorptivities of major hemoglobin derivatives•This approach fills a current need for the accurate quantification of methemoglobin in fishes possessing unstable hemoglobin.
Keywords: Coho salmon; Fish hemoglobin; Hemichrome; Methemoglobin; Multi-component analysis; Unstable hemoglobin.
© 2020 The Authors. Published by Elsevier B.V.
Figures


Similar articles
-
Identification of the sex chromosome pair in chum salmon (Oncorhynchus keta) and pink salmon (Oncorhynchus gorbuscha).Cytogenet Genome Res. 2007;116(4):298-304. doi: 10.1159/000100414. Cytogenet Genome Res. 2007. PMID: 17431328
-
Xenoma formation during microsporidial gill disease of salmonids caused by Loma salmonae is affected by host species (Oncorhynchus tshawytscha, O. kisutch, O. mykiss) but not by salinity.Dis Aquat Organ. 2002 Mar 11;48(2):125-31. doi: 10.3354/dao048125. Dis Aquat Organ. 2002. PMID: 12005234
-
Acute Toxicity of 6PPD-Quinone to Early Life Stage Juvenile Chinook (Oncorhynchus tshawytscha) and Coho (Oncorhynchus kisutch) Salmon.Environ Toxicol Chem. 2023 Apr;42(4):815-822. doi: 10.1002/etc.5568. Environ Toxicol Chem. 2023. PMID: 36692118
-
Identification of the sex chromosome pair in coho salmon (Oncorhynchus kisutch): lack of conservation of the sex linkage group with chinook salmon (Oncorhynchus tshawytscha).Cytogenet Genome Res. 2005;111(2):166-70. doi: 10.1159/000086387. Cytogenet Genome Res. 2005. PMID: 16103659
-
Piscine orthoreovirus: Biology and distribution in farmed and wild fish.J Fish Dis. 2020 Nov;43(11):1331-1352. doi: 10.1111/jfd.13228. Epub 2020 Sep 15. J Fish Dis. 2020. PMID: 32935367 Review.
Cited by
-
Artificial blood-hope and the challenges to combat tumor hypoxia for anti-cancer therapy.Med Biol Eng Comput. 2025 Apr;63(4):933-957. doi: 10.1007/s11517-024-03233-6. Epub 2024 Nov 30. Med Biol Eng Comput. 2025. PMID: 39614063 Review.
References
-
- Scholz N.L., Myers M.S., McCarthy S.G., Labenia J.S., McIntyre J.K., Ylitalo G.M., Rhodes L.D., Laetz C.A., Stehr C.M., French B.L., McMillan B., Wilson D., Reed L., Lynch K., Damm S., Davis J., McMillan B. Recurrent die-offs of adult coho salmon returning to spawn in puget sound lowland urban streams. PLoS ONE. 2011;6(12):e28013. - PMC - PubMed
-
- Zijlstra W.G., Buursma A., van Assendelft O.W., editors. Visible and Near Infrared Absorption Spectra of Human and Animal hemoglobin: Determination and Application. VSP; 2000.
-
- Alam M.K., Callis J.B. Elucidation of species in alcohol-water mixtures using near-IR spectroscopy and multivariate statistics. Anal. Chem. 1994;66(14):2293–2301.
-
- Rachmilewitz E.A. Formation of hemichromes from oxidized hemoglobin subunits. Ann. N. Y. Acad. Sci. 1969;165(1):171–184. - PubMed
-
- Riggs A. Vol. 76. Academic Press; 1981. Preparation of blood hemoglobins of vertebrates; pp. 5–29. (Methods in Enzymology). - PubMed
LinkOut - more resources
Full Text Sources

