Arylquin 1, a potent Par-4 secretagogue, induces lysosomal membrane permeabilization-mediated non-apoptotic cell death in cancer cells
- PMID: 32257929
- PMCID: PMC7099120
- DOI: 10.1007/s43188-019-00025-1
Arylquin 1, a potent Par-4 secretagogue, induces lysosomal membrane permeabilization-mediated non-apoptotic cell death in cancer cells
Abstract
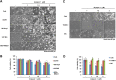
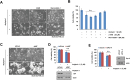
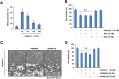
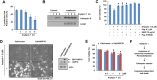
Arylquin 1, a small-molecule prostate-apoptosis-response-4 (Par-4) secretagogue, targets vimentin to induce Par-4 secretion. Secreted Par-4 binds to its receptor, 78-kDa glucose-regulated protein (GRP78), on the cancer cell surface and induces apoptosis. In the present study, we investigated the molecular mechanisms of arylquin 1 in cancer cell death. Arylquin 1 induces morphological changes (cell body shrinkage and cell detachment) and decreases cell viability in various cancer cells. Arylquin 1-induced cell death is not inhibited by apoptosis inhibitors (z-VAD-fmk, a pan-caspase inhibitor), necroptosis inhibitors (necrostatin-1), and paraptosis inhibitors. Furthermore, arylquin 1 significantly induces reactive oxygen species levels, but antioxidants [N-acetyl-l-cysteine and glutathione ethyl ester] do not inhibit arylquin 1-induced cell death. Furthermore, Par-4 knock-down by small interfering RNA confers no effect on cytotoxicity in arylquin 1-treated cells. Interestingly, arylquin 1 induces lysosomal membrane permeabilization (LMP), and cathepsin inhibitors and overexpression of 70-kDa heat shock protein (HSP70) markedly prevent arylquin 1-induced cell death. Therefore, our results suggest that arylquin 1 induces non-apoptotic cell death in cancer cells through the induction of LMP.
Keywords: Arylquin 1; Cell death; Lysosomal membrane permeabilization; Non-apoptotic cell death; Prostate‐apoptosis‐response‐4.
© Korean Society of Toxicology 2019.
Conflict of interest statement
Conflict of interestThe authors declare no conflicts of interest.
Figures
References
-
- Sells SF, Wood DP, Jr, Joshi-Barve SS, Muthukumar S, Jacob RJ, Crist SA, Humphreys S, Rangnekar VM. Commonality of the gene programs induced by effectors of apoptosis in androgen-dependent and -independent prostate cells. Cell Growth Differ. 1994;5:457–466. - PubMed
-
- Chakraborty M, Qiu SG, Vasudevan KM, Rangnekar VM. Par-4 drives trafficking and activation of Fas and Fasl to induce prostate cancer cell apoptosis and tumor regression. Can Res. 2001;61:7255–7263. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

