Different Effects of Pre-transplantation Measurable Residual Disease on Outcomes According to Transplant Modality in Patients With Philadelphia Chromosome Positive ALL
- PMID: 32257948
- PMCID: PMC7089930
- DOI: 10.3389/fonc.2020.00320
Different Effects of Pre-transplantation Measurable Residual Disease on Outcomes According to Transplant Modality in Patients With Philadelphia Chromosome Positive ALL
Abstract
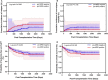
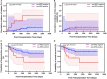
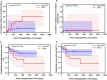
Background: This study compared the effects of pre-transplantation measurable residual disease (pre-MRD) on outcomes in Philadelphia chromosome (Ph)-positive ALL patients who underwent human leukocyte antigen-matched sibling donor transplantation (MSDT) or who received unmanipulated haploidentical SCT (haplo-SCT). Methods: A retrospective study (n = 202) was performed. MRD was detected by RT-PCR and multiparameter flow cytometry. Results: In the total patient group, patients with positive pre-MRD had a higher 4-year cumulative incidence of relapse (CIR) than that in patients with negative pre-MRD (26.1% vs. 12.1%, P = 0.009); however, the cumulative incidence of non-relapse mortality (NRM) (7.4% vs. 15.9%, P = 0.148), probability of leukemia-free survival (LFS) (66.3% vs. 71.4%, P = 0.480), and overall survival (OS) (68.8% vs. 76.5%, P = 0.322) were comparable. In the MSDT group, patients with positive pre-MRD had increased 4-year CIR (56.4% vs. 13.8%, P < 0.001) and decreased 4-year LFS (35.9% vs. 71.0%, P = 0.024) and OS (35.9% vs. 77.6%, P = 0.011) compared with those with negative pre-MRD. In haplo-SCT settings, the 4-year CIR (14.8% vs. 10.7%, P = 0.297), NRM (7.3% vs. 16.3%, P = 0.187) and the 4-year probability of OS (77.7% vs. 72.3%, P = 0.804) and LFS (80.5% vs. 75.7%, P = 0.660) were comparable between pre-MRD positive and negative groups. In subgroup patients with positive pre-MRD, haplo-SCT had a lower 4-year CIR (14.8% vs. 56.4%, P = 0.021) and a higher 4-year LFS (77.7% vs. 35.9%, P = 0.036) and OS (80.5% vs. 35.9%, P = 0.027) than those of MSDT. Multivariate analysis showed that haplo-SCT was associated with lower CIR (HR, 0.288; P = 0.031), superior LFS (HR, 0.283; P = 0.019) and OS (HR, 0.252; P = 0.013) in cases with a positive pre-MRD subgroup. Conclusions: Our results indicate that the effects of positive pre-MRD on the outcomes of patients with Ph-positive ALL are different according to transplant modality. For Ph-positive cases with positive pre-MRD, haplo-SCT might have strong graft-vs.-leukemia (GVL) effects.
Keywords: HLA-matched sibling donor transplantation; Philadelphia-chromosome positive; acute lymphoblastic leukemia; haploidentical allografts; measurable residual disease.
Copyright © 2020 Li, Fan, Xu, Wang, Zhang, Chen, Chen, Wang, Han, Sun, Yan, Tang, Liu, Mo, Wang, Liu, Huang and Chang.
Figures
References
-
- Eckert C, Henze G, Seeger K, Hagedorn N, Mann G, Panzer-Grumayer R, et al. . Use of allogeneic hematopoietic stem-cell transplantation based on minimal residual disease response improves outcomes for children with relapsed acute lymphoblastic leukemia in the intermediate-risk group. J Clin Oncol Offi J Am Soc Clin Oncol. (2013) 31:2736–42. 10.1200/JCO.2012.48.5680 - DOI - PubMed
-
- Daver N, Thomas D, Ravandi F, Cortes J, Garris R, Jabbour E, et al. . Final report of a phase II study of imatinib mesylate with hyper-CVAD for the front-line treatment of adult patients with Philadelphia chromosome-positive acute lymphoblastic leukemia. Haematologica. (2015) 100:653–61. 10.3324/haematol.2014.118588 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

