Diverse Role of TGF-β in Kidney Disease
- PMID: 32258028
- PMCID: PMC7093020
- DOI: 10.3389/fcell.2020.00123
Diverse Role of TGF-β in Kidney Disease
Abstract
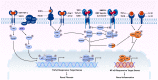
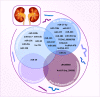
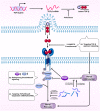
Inflammation and fibrosis are two pathological features of chronic kidney disease (CKD). Transforming growth factor-β (TGF-β) has been long considered as a key mediator of renal fibrosis. In addition, TGF-β also acts as a potent anti-inflammatory cytokine that negatively regulates renal inflammation. Thus, blockade of TGF-β inhibits renal fibrosis while promoting inflammation, revealing a diverse role for TGF-β in CKD. It is now well documented that TGF-β1 activates its downstream signaling molecules such as Smad3 and Smad3-dependent non-coding RNAs to transcriptionally and differentially regulate renal inflammation and fibrosis, which is negatively regulated by Smad7. Therefore, treatments by rebalancing Smad3/Smad7 signaling or by specifically targeting Smad3-dependent non-coding RNAs that regulate renal fibrosis or inflammation could be a better therapeutic approach. In this review, the paradoxical functions and underlying mechanisms by which TGF-β1 regulates in renal inflammation and fibrosis are discussed and novel therapeutic strategies for kidney disease by targeting downstream TGF-β/Smad signaling and transcriptomes are highlighted.
Keywords: Smads; TGF-β; fibrosis; inflammation; mechanisms; therapy.
Copyright © 2020 Gu, Liu, Huang, Yu and Lan.
Figures

References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

