Aristolochic Acid-Induced Genotoxicity and Toxicogenomic Changes in Rodents
- PMID: 32258091
- PMCID: PMC7110418
- DOI: 10.4103/wjtcm.wjtcm_33_19
Aristolochic Acid-Induced Genotoxicity and Toxicogenomic Changes in Rodents
Abstract
Aristolochic acid (AA) is a group of structurally related nitrophenanthrene carboxylic acids found in many plants that are widely used by many cultures as traditional herbal medicines. AA is a causative agent for Chinese herbs nephropathy, a term replaced later by AA nephropathy. Evidence indicates that AA is nephrotoxic, genotoxic, and carcinogenic in humans; and it also induces tumors in the forestomach, kidney, renal pelvis, urinary bladder, and lung of rats and mice. Therefore, plants containing AA have been classified as carcinogenic to humans (Group 1) by the International Agency for Research on Cancer. In our laboratories, we have conducted a series of genotoxicity and toxicogenomic studies in the rats exposed to AA of 0.1-10 mg/kg for 12 weeks. Our results demonstrated that AA treatments induced DNA adducts and mutations in the kidney, liver, and spleen of rats, as well as significant alteration of gene expression in both its target and nontarget tissues. AA treatments altered mutagenesis- or carcinogenesis-related microRNA expression in rat kidney and resulted in significant changes in protein expression profiling. We also applied benchmark dose (BMD) modeling to the 3-month AA-induced genotoxicity data. The obtained BMDL10 (the lower 95% confidence interval of the BMD10 that is a 10% increase over the background level) for AA-induced mutations in the kidney of rats was about 7 μg/kg body weight per day. This review constitutes an overview of our investigations on AA-induced genotoxicity and toxicogenomic changes including gene expression, microRNA expression, and proteomics; and presents updated information focused on AA-induced genotoxicity in rodents.
Keywords: Aristolochic acid; benchmark dose; genotoxicity; mutation; toxicogenomics.
Conflict of interest statement
Conflicts of interest There are no conflicts of interest.
Figures




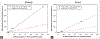
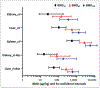
References
-
- National Toxicology Program. Final report on carcinogens background document for aristolochic acids. Rep Carcinog Backgr Doc 2008;5976:1–246. - PubMed
-
- Luciano RL, Perazella MA. Aristolochic acid nephropathy: Epidemiology, clinical presentation, and treatment. Drug Saf 2015;38:55–64. - PubMed
-
- Holden F, Amin V, Kuek D, Kopp JB, Hendry BM, Xu Q. Taming the fire of nephrotoxic botanicals. World J Tradit Chin Med 2019;5:151–63.
-
- IARC. Plants containing aristolochic acid. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans 2012. Lyon, France: IARC 100A; 2012. Available from: https://monographs.iarc.fr/wp-content/uploads/2018/06/mono100A-23.pdf. [Last accessed on 2019 Jun 20].
-
- Heinrich M, Chan J, Wanke S, Neinhuis C, Simmonds MS. Local uses of Aristolochia species and content of nephrotoxic aristolochic acid 1 and 2 – A global assessment based on bibliographic sources. J Ethnopharmacol 2009;125:108–44. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
