Transcriptome Analysis Reveals Key Pathways and Hormone Activities Involved in Early Microtuber Formation of Dioscorea opposita
- PMID: 32258146
- PMCID: PMC7086419
- DOI: 10.1155/2020/8057929
Transcriptome Analysis Reveals Key Pathways and Hormone Activities Involved in Early Microtuber Formation of Dioscorea opposita
Abstract
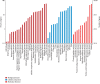
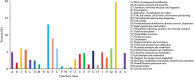
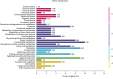
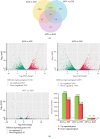
Chinese yam (Dioscorea opposita) is an important tuberous crop used for both food and medicine. Despite a long history of cultivation, the understanding of D. opposita genetics and molecular biology remains scant, which has limited its genetic improvement. This work presents a de novo transcriptome sequencing analysis of microtuber formation in D. opposita. We assembled cDNA libraries from different stages during the process of microtuber formation, designated as initial explants (EXP), axillary bud proliferation after three weeks (BUD), and microtuber visible after four weeks (MTV). More differentially expressed genes (DEGs) and pathways were identified between BUD vs. EXP than in MTV vs. BUD, indicating that proliferation of the axillary bud is the key stage of microtuber induction. Gene classification and pathway enrichment analysis showed that microtuber formation is tightly coordinated with primary metabolism, such as amino acid biosynthesis, ribosomal component biosynthesis, and starch and sucrose metabolism. The formation of the microtuber is regulated by a variety of plant hormones, including ABA. Combined with analysis of physiological data, we suggest that ABA positively regulates tuberization in D. opposita. This study will serve as an empirical foundation for future molecular studies and for the propagation of D. opposita germplasm in field crops.
Copyright © 2020 Junhua Li et al.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

Similar articles
-
A protocol for in vitro production of microtubers in Chinese yam (Dioscorea opposita).Biosci Biotechnol Biochem. 2014;78(6):1005-9. doi: 10.1080/09168451.2014.912119. Epub 2014 May 28. Biosci Biotechnol Biochem. 2014. PMID: 25036126
-
Integrated mRNA and miRNA transcriptome analysis reveals a regulatory network for tuber expansion in Chinese yam (Dioscorea opposita).BMC Genomics. 2020 Feb 3;21(1):117. doi: 10.1186/s12864-020-6492-5. BMC Genomics. 2020. PMID: 32013881 Free PMC article.
-
[Variation of endogenous hormones in formation of microtuber of Dioscorea opposite in vitro].Zhongguo Zhong Yao Za Zhi. 2010 Nov;35(21):2818-21. Zhongguo Zhong Yao Za Zhi. 2010. PMID: 21322938 Chinese.
-
[Preliminary study of Dioscorea bulbifera plantlet microtuber in vitro induction].Zhong Yao Cai. 2014 Apr;37(4):543-7. Zhong Yao Cai. 2014. PMID: 25345120 Chinese.
-
A Frontier Review of Nutraceutical Chinese Yam.Foods. 2024 May 7;13(10):1426. doi: 10.3390/foods13101426. Foods. 2024. PMID: 38790726 Free PMC article. Review.
Cited by
-
Identification of BBX gene family and its function in the regulation of microtuber formation in yam.BMC Genomics. 2023 Jun 26;24(1):354. doi: 10.1186/s12864-023-09406-1. BMC Genomics. 2023. PMID: 37365511 Free PMC article.
-
Bulbil initiation: a comprehensive review on resources, development, and utilisation, with emphasis on molecular mechanisms, advanced technologies, and future prospects.Front Plant Sci. 2024 Apr 8;15:1343222. doi: 10.3389/fpls.2024.1343222. eCollection 2024. Front Plant Sci. 2024. PMID: 38650701 Free PMC article. Review.
-
Transcriptome Profiling Reveals Differential Gene Expression during the Process of Microtuber Formation in Pinellia ternata.Int J Mol Sci. 2023 Jul 18;24(14):11604. doi: 10.3390/ijms241411604. Int J Mol Sci. 2023. PMID: 37511363 Free PMC article.
-
DoDELLA-GAI2 Integrates Gibberellin and Ethylene Signaling to Regulate Chinese Yam (Dioscorea opposita) Tuber Development.Biology (Basel). 2025 May 30;14(6):635. doi: 10.3390/biology14060635. Biology (Basel). 2025. PMID: 40563886 Free PMC article.
-
Roles of Abscisic Acid and Gibberellins in Stem/Root Tuber Development.Int J Mol Sci. 2022 Apr 29;23(9):4955. doi: 10.3390/ijms23094955. Int J Mol Sci. 2022. PMID: 35563355 Free PMC article. Review.
References
-
- Cheng M., Wang D., Peng H. Evolution and change of the species quality and authentic producing areas of Dioscorea opposita Thunb. Chinese Journal of Medical History. 2014;44(2):81–84. - PubMed
-
- Borges M., Ceiro W., Meneses S., et al. Regeneration and multiplication of Dioscorea alata germplasm maintained in vitro. Plant Cell, Tissue and Organ Culture. 2004;76(1):87–90. doi: 10.1023/A:1025804516226. - DOI
-
- Malaurie B., Pungu O., Trouslot M.-F. Effect of growth regulators concentrations on morphological development of meristem-tips in Dioscorea cayenensis-D. rotundata complex and D. praehensilis. Plant Cell Tissue and Organ Culture. 1995;41(3):229–235. doi: 10.1007/BF00045086. - DOI
-
- Ovono P. O., Kevers C., Dommes J. Axillary proliferation and tuberisation of Dioscorea cayenensis–D. rotundata complex. Plant Cell Tissue and Organ Culture. 2007;91(2):107–114. doi: 10.1007/s11240-007-9238-z. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

