Robotically Steered Needles: A Survey of Neurosurgical Applications and Technical Innovations
- PMID: 32258180
- PMCID: PMC7090177
- DOI: 10.2147/RSRR.S224446
Robotically Steered Needles: A Survey of Neurosurgical Applications and Technical Innovations
Abstract
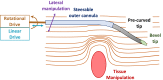
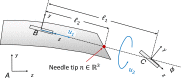
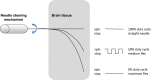
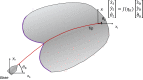
This paper surveys both the clinical applications and main technical innovations related to steered needles, with an emphasis on neurosurgery. Technical innovations generally center on curvilinear robots that can adopt a complex path that circumvents critical structures and eloquent brain tissue. These advances include several needle-steering approaches, which consist of tip-based, lengthwise, base motion-driven, and tissue-centered steering strategies. This paper also describes foundational mathematical models for steering, where potential fields, nonholonomic bicycle-like models, spring models, and stochastic approaches are cited. In addition, practical path planning systems are also addressed, where we cite uncertainty modeling in path planning, intraoperative soft tissue shift estimation through imaging scans acquired during the procedure, and simulation-based prediction. Neurosurgical scenarios tend to emphasize straight needles so far, and span deep-brain stimulation (DBS), stereoelectroencephalography (SEEG), intracerebral drug delivery (IDD), stereotactic brain biopsy (SBB), stereotactic needle aspiration for hematoma, cysts and abscesses, and brachytherapy as well as thermal ablation of brain tumors and seizure-generating regions. We emphasize therapeutic considerations and complications that have been documented in conjunction with these applications.
Keywords: brain biopsy; brachytherapy; deep-brain stimulation; intracerebral drug delivery; needle steering; stereoelectroencephalography; stereotactic needle aspiration.
© 2020 Audette et al.
Conflict of interest statement
The authors report no conflicts of interest in this work.
Figures






Similar articles
-
Needle-tissue interactive mechanism and steering control in image-guided robot-assisted minimally invasive surgery: a review.Med Biol Eng Comput. 2018 Jun;56(6):931-949. doi: 10.1007/s11517-018-1825-0. Epub 2018 Apr 21. Med Biol Eng Comput. 2018. PMID: 29679255 Review.
-
Magnetic Control of a Flexible Needle in Neurosurgery.IEEE Trans Biomed Eng. 2021 Feb;68(2):616-627. doi: 10.1109/TBME.2020.3009693. Epub 2021 Jan 20. IEEE Trans Biomed Eng. 2021. PMID: 32746060
-
Evaluation of Robotic Needle Steering in ex vivo Tissue.IEEE Int Conf Robot Autom. 2010 May 3;2010:2068-2073. doi: 10.1109/ROBOT.2010.5509873. IEEE Int Conf Robot Autom. 2010. PMID: 21339851 Free PMC article.
-
3D path planning for flexible needle steering in neurosurgery.Int J Med Robot. 2019 Aug;15(4):e1998. doi: 10.1002/rcs.1998. Epub 2019 May 9. Int J Med Robot. 2019. PMID: 30945791
-
Flexible Needle Steering with Tethered and Untethered Actuation: Current States, Targeting Errors, Challenges and Opportunities.Ann Biomed Eng. 2023 May;51(5):905-924. doi: 10.1007/s10439-023-03163-8. Epub 2023 Mar 21. Ann Biomed Eng. 2023. PMID: 36943414 Review.
Cited by
-
A Survey of Needle Steering Approaches in Minimally Invasive Surgery.Ann Biomed Eng. 2024 Jun;52(6):1492-1517. doi: 10.1007/s10439-024-03494-0. Epub 2024 Mar 26. Ann Biomed Eng. 2024. PMID: 38530535 Review.
-
Insights into Infusion-Based Targeted Drug Delivery in the Brain: Perspectives, Challenges and Opportunities.Int J Mol Sci. 2022 Mar 15;23(6):3139. doi: 10.3390/ijms23063139. Int J Mol Sci. 2022. PMID: 35328558 Free PMC article. Review.
References
Publication types
LinkOut - more resources
Full Text Sources

