Safety and Outcomes of Linezolid Use for Nocardiosis
- PMID: 32258209
- PMCID: PMC7112726
- DOI: 10.1093/ofid/ofaa090
Safety and Outcomes of Linezolid Use for Nocardiosis
Abstract
Background: Tropical Australia has a high incidence of nocardiosis, with high rates of intrinsic antimicrobial resistance. Linezolid, the only antimicrobial to which all local Nocardia species are susceptible, has been recommended in empirical combination treatment regimens for moderate-severe Nocardia infections at Royal Darwin Hospital (RDH) since 2014. We report the safety and efficacy of linezolid use for nocardiosis in this setting.
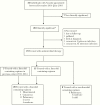
Methods: We identified cases through a retrospective review of all RDH Nocardia isolates from December 2014 to August 2018 and included 5 linezolid-treated cases from a previous cohort. Laboratory, demographic, and clinical data were included in the primary analysis of safety and treatment outcomes.
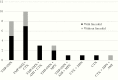
Results: Between 2014 and 2018, Nocardia was isolated from 35 individuals; 28 (80%) had clinically significant infection and 23 (82%) received treatment. All isolates were linezolid-susceptible. Safety and efficacy were assessed for 20 patients receiving linezolid-containing regimens and 8 receiving nonlinezolid regimens. Median linezolid induction therapy duration was 28 days. Common adverse effects in those receiving linezolid were thrombocytopenia (45%) and anemia (40%). Adverse events prompted discontinuation of trimethoprim-sulfamethoxazole more often than linezolid (40% vs 20%). Linezolid therapeutic drug monitoring was used in 1 patient, with successful dose reduction and outcome. There was no difference in 30-day survival between those treated with linezolid (90%) vs no linezolid (87%). One Nocardia-attributed death occurred during linezolid therapy.
Conclusions: Linezolid is safe and efficacious in empirical treatment for moderate to severe nocardiosis in a monitored hospital setting, with 100% drug susceptibility and no difference in adverse events or outcomes compared with nonlinezolid regimens.
Keywords: Nocardia; antimicrobial resistance; linezolid; prophylaxis; therapy.
© The Author(s) 2020. Published by Oxford University Press on behalf of Infectious Diseases Society of America.
Figures

References
-
- Fatahi-Bafghi M. Nocardiosis from 1888 to 2017. Microb Pathog 2018; 114:369–84. - PubMed
-
- Sorell TC MItchell DH, Iredell JR, Chen SC. Nocardia spp. In: Mandell GL, Bennett JE, Dolin R, eds. Principles and Practice of Infectious Diseases. 8th ed.Philadelphia: Elsevier; 2016:2853.
-
- Peleg AY, Husain S, Qureshi ZA, et al. Risk factors, clinical characteristics, and outcome of Nocardia infection in organ transplant recipients: a matched case-control study. Clin Infect Dis 2007; 44:1307–14. - PubMed
-
- Mamelak AN, Obana WG, Flaherty JF, Rosenblum ML. Nocardial brain abscess: treatment strategies and factors influencing outcome. Neurosurgery 1994; 35:622–31. - PubMed

