Defining the Optimal FVIII Transgene for Placental Cell-Based Gene Therapy to Treat Hemophilia A
- PMID: 32258210
- PMCID: PMC7109377
- DOI: 10.1016/j.omtm.2020.03.001
Defining the Optimal FVIII Transgene for Placental Cell-Based Gene Therapy to Treat Hemophilia A
Abstract
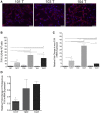
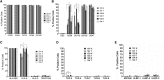
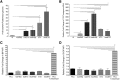
The delivery of factor VIII (FVIII) through gene and/or cellular platforms has emerged as a promising hemophilia A treatment. Herein, we investigated the suitability of human placental cells (PLCs) as delivery vehicles for FVIII and determined an optimal FVIII transgene to produce/secrete therapeutic FVIII levels from these cells. Using three PLC cell banks we demonstrated that PLCs constitutively secreted low levels of FVIII, suggesting their suitability as a transgenic FVIII production platform. Furthermore, PLCs significantly increased FVIII secretion after transduction with a lentiviral vector (LV) encoding a myeloid codon-optimized bioengineered FVIII containing high-expression elements from porcine FVIII. Importantly, transduced PLCs did not upregulate cellular stress or innate immunity molecules, demonstrating that after transduction and FVIII production/secretion, PLCs retained low immunogenicity and cell stress. When LV encoding five different bioengineered FVIII transgenes were compared for transduction efficiency, FVIII production, and secretion, data showed that PLCs transduced with LV encoding hybrid human/porcine FVIII transgenes secreted substantially higher levels of FVIII than did LV encoding B domain-deleted human FVIII. In addition, data showed that in PLCs, myeloid codon optimization is needed to increase FVIII secretion to therapeutic levels. These studies have identified an optimal combination of FVIII transgene and cell source to achieve clinically meaningful levels of secreted FVIII.
Keywords: ET3; FVIII; HSQ; cell therapy; codon-optimization; gene therapy; hemophilia A; placental cells.
© 2020 The Author(s).
Figures


Similar articles
-
Comparison of different gene addition strategies to modify placental derived-mesenchymal stromal cells to produce FVIII.Front Immunol. 2022 Dec 15;13:954984. doi: 10.3389/fimmu.2022.954984. eCollection 2022. Front Immunol. 2022. PMID: 36591257 Free PMC article.
-
Preclinical Development of a Hematopoietic Stem and Progenitor Cell Bioengineered Factor VIII Lentiviral Vector Gene Therapy for Hemophilia A.Hum Gene Ther. 2018 Oct;29(10):1183-1201. doi: 10.1089/hum.2018.137. Hum Gene Ther. 2018. PMID: 30160169 Free PMC article.
-
Generation of an optimized lentiviral vector encoding a high-expression factor VIII transgene for gene therapy of hemophilia A.Gene Ther. 2013 Jun;20(6):607-15. doi: 10.1038/gt.2012.76. Epub 2012 Sep 20. Gene Ther. 2013. PMID: 22996197 Free PMC article.
-
Hemophilia A gene therapy via intraosseous delivery of factor VIII-lentiviral vectors.Thromb J. 2016 Oct 4;14(Suppl 1):41. doi: 10.1186/s12959-016-0105-1. eCollection 2016. Thromb J. 2016. PMID: 27766066 Free PMC article. Review.
-
Development of improved factor VIII molecules and new gene transfer approaches for hemophilia A.Curr Gene Ther. 2003 Feb;3(1):27-41. doi: 10.2174/1566523033347417. Curr Gene Ther. 2003. PMID: 12553533 Review.
Cited by
-
Investigating the impact of synonymous gene recoding on a recombinantly expressed monoclonal antibody under different process parameters.Bioeng Transl Med. 2025 Jan 27;10(3):e10750. doi: 10.1002/btm2.10750. eCollection 2025 May. Bioeng Transl Med. 2025. PMID: 40385532 Free PMC article.
-
In Vitro Conditioning of Adipose-Derived Mesenchymal Stem Cells by the Endothelial Microenvironment: Modeling Cell Responsiveness towards Non-Genetic Correction of Haemophilia A.Int J Mol Sci. 2022 Jun 30;23(13):7282. doi: 10.3390/ijms23137282. Int J Mol Sci. 2022. PMID: 35806285 Free PMC article.
-
Hemophilia A: An Ideal Disease for Prenatal Therapy.Prenat Diagn. 2025 Jun 10:10.1002/pd.6833. doi: 10.1002/pd.6833. Online ahead of print. Prenat Diagn. 2025. PMID: 40495292 Review.
-
Effects of Shear Stress on Production of FVIII and vWF in a Cell-Based Therapeutic for Hemophilia A.Front Bioeng Biotechnol. 2021 Mar 1;9:639070. doi: 10.3389/fbioe.2021.639070. eCollection 2021. Front Bioeng Biotechnol. 2021. PMID: 33732691 Free PMC article.
-
Transplanting FVIII/ET3-secreting cells in fetal sheep increases FVIII levels long-term without inducing immunity or toxicity.Nat Commun. 2023 Jul 14;14(1):4206. doi: 10.1038/s41467-023-39986-1. Nat Commun. 2023. PMID: 37452013 Free PMC article.
References
-
- Rangarajan S., Walsh L., Lester W., Perry D., Madan B., Laffan M., Yu H., Vettermann C., Pierce G.F., Wong W.Y., Pasi K.J. AAV5-factor VIII gene transfer in severe hemophilia A. N. Engl. J. Med. 2017;377:2519–2530. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

