Temperature-dependent formulation of a hydrogel based on Hyaluronic acid-polydimethylsiloxane for biomedical applications
- PMID: 32258450
- PMCID: PMC7096762
- DOI: 10.1016/j.heliyon.2020.e03494
Temperature-dependent formulation of a hydrogel based on Hyaluronic acid-polydimethylsiloxane for biomedical applications
Abstract
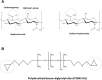
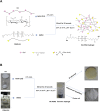
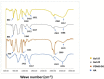
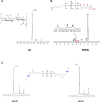
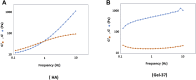
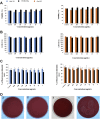
Hyaluronic acid (HA), as a safe biomaterial with minimal immunogenicity, is being employed in a broad range of medical applications. Since unmodified HA has a high potential for biodegradation in the physiological condition, herein, an HA-based cross-linked hydrogel was formulated using polydimethylsiloxane-diglycidyl ether terminated (PDMS-DG) via epoxide-OH reaction. The formation of HA-PDMS hydrogel was confirmed using FTIR, NMR, and FESEM. Temperature demonstrated a critical role in the physicochemical properties of the final products. Gel-37, which formed at 37 °C, had a higher modification degree (MD) and more stability against hyaluronidase and oxidative stress than the hydrogel formulated at 25 °C (Gel-25). In addition, the swelling ratio, roughness, and porous network topology of Gel-25 and Gel-37 were different. The rheology measurement indicated that HA-PDMS hydrogel had a stable viscoelastic character. The hydrogel was also biocompatible, non-cytotoxic, and considerably stable during 7-months storage. Overall, various determined parameters confirmed that HA-PDMS hydrogel is worth using in different medical applications. Keywords: Hyaluronic acid; Polydimethylsiloxane-diglycidyl ether terminated; Hydrogels; Long-term stability; Viscoelastic behavior; Biocompatibility.
Keywords: Biocompatibility; Hyaluronic acid; Hydrogels; Long-term stability; Materials science; Polydimethylsiloxane-diglycidyl ether terminated; Viscoelastic behavior.
© 2020 Published by Elsevier Ltd.
Figures




References
-
- Al-Sibani M., Al-Harrasi A., Neubert R.H. Study of the effect of mixing approach on cross-linking efficiency of hyaluronic acid-based hydrogel cross-linked with 1, 4-butanediol diglycidyl ether. Eur. J. Pharmaceut. Sci. 2016;91:131–137. - PubMed
-
- Schanté C.E., Zuber G., Herlin C., Vandamme T.F. Chemical modifications of hyaluronic acid for the synthesis of derivatives for a broad range of biomedical applications. Carbohydr. Polym. 2011;85:469–489.
-
- Kablik J., Monheit G.D., Yu L., Chang G., Gershkovich J. Comparative physical properties of hyaluronic acid dermal fillers. Dermatol. Surg. 2009;35:302–312. - PubMed
-
- Robert L., Robert A.-M., Renard G. Biological effects of hyaluronan in connective tissues, eye, skin, venous wall. Role in aging. Pathol. Biol. 2010;58:187–198. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

