Expression analysis of glutathione S-transferases and ferritins during the embryogenesis of the tick Haemaphysalis longicornis
- PMID: 32258487
- PMCID: PMC7114739
- DOI: 10.1016/j.heliyon.2020.e03644
Expression analysis of glutathione S-transferases and ferritins during the embryogenesis of the tick Haemaphysalis longicornis
Abstract
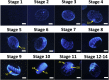
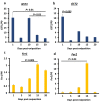
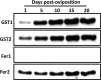
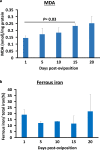
In the tick life cycle, embryogenesis is the only stage of development wherein no blood meal is required. Nevertheless, even in the absence of a blood meal, which is the source of nutrients as well as the ferrous iron and heme that could cause oxidative stress in ticks, malondialdehyde (MDA) has been reported to increase during this period. Additionally, the knockdown of some oxidative stress-related molecules such as ferritin has resulted in abnormal eggs and embryonic death. Here, we investigate the gene and protein expression profiles of the identified glutathione S-transferases (GSTs) and ferritins (Fers) of the tick H. longicornis during embryogenesis through quantitative reverse transcription polymerase chain reaction (RT-qPCR) and Western blotting, respectively. We also confirm the lipid peroxidation and ferrous iron concentration level using a thiobarbituric acid reactive substances (TBARS) assay. Finally, we attempt to correlate these findings with the events occurring by establishing a staging process in H. longicornis embryos. Lipid peroxidation increased during the course of embryogenesis, as does the amount of GST proteins. On the other hand, the GST genes have high expression at the 1st day post-oviposition, during the early stage of embryogenesis and at day 10 during the period wherein the germ band is observable. Fer gene expression also starts to increase at day 10 and peaks at day 15. In the ferritin proteins, only the secretory ferritin (Fer2) is detected and constitutively expressed during embryogenesis. Events occurring during embryogenesis, such as energy production and iron metabolism for cellular proliferation and differentiation cause oxidative stress in the embryo. To counteract oxidative stress, it is possible that the embryo may utilize oxidative stress-related molecules such as GSTs and Fer2, which could be either maternally or embryo-derived.
Keywords: Developmental biology; Embryology; Genomics; Oxidative stress; Proteomics; Transcriptome; Veterinary medicine.
© 2020 The Author(s).
Figures
References
-
- Ali A., Khan M.A., Zahid H., Yaseen P.M., Qayash-Khan M., Nawab J., Ur-Rehman Z., Ateeq M., Khan S., Ibrahim M. Seasonal dynamics, record of ticks infesting humans, wild and domestic animals and molecular phylogeny of Rhipicephalus microplus in Khyber Pakhtunkhwa Pakistan. Front. Physiol. 2019;10:793. - PMC - PubMed
-
- Campos E., Façanha A., Moraes J., da Silva Vaz I., Masuda A., Logullo C. A mitochondrial exopolyphosphatase activity modulated by phosphate demand in Rhipicephalus (Boophilus) microplus embryo. Insect Biochem. Mol. Biol. 2007;37:1103–1107. - PubMed
-
- Campos E., Façanha A.R., Costa E.P., Fraga A., Moraes J., da Silva Vaz I., Masuda A., Logullo C. A mitochondrial membrane exopolyphosphatase is modulated by, and plays a role in, the energy metabolism of hard tick Rhipicephalus (Boophilus) microplus embryos. Int. J. Mol. Sci. 2011;12:3525–3535. - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

