Synthesis of new functionalized thiazolo pyridine-fused and thiazolo pyridopyrimidine-fused spirooxindoles via one-pot reactions
- PMID: 32258502
- PMCID: PMC7114753
- DOI: 10.1016/j.heliyon.2020.e03687
Synthesis of new functionalized thiazolo pyridine-fused and thiazolo pyridopyrimidine-fused spirooxindoles via one-pot reactions
Abstract
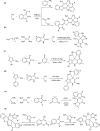
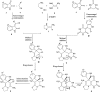
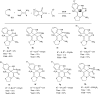
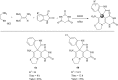
A sequential multi-component reaction of the nitroketene dithioacetals, cysteamine hydrochloride, isatin and different CH-acids is described. This efficient method provides new functionalized thiazolo pyridine-fused spirooxindoles and thiazolo pyridopyrimidine-fused spirooxindoles in good yields. In the case of using isatin derivatives (5-bromoisatin and 5-chloroisatin), the reaction was carried out by using nano-SiO2 (20 mol%) as an effective heterogeneous Lewis acid promoter. This type of reaction provides a range of skeletally different polycyclic spiro thiazole-based heterocyclic structures and represents attractive advantages including straightforward one-pot operation under the catalyst-free condition and simple workup procedures without using tedious purification procedure.
Keywords: Isatin; Nano-SiO2; Nitroketene dithioacetals; Organic chemistry; Spiro heterocycles; Spirooxindoles.
© 2020 The Authors. Published by Elsevier Ltd.
Figures




Similar articles
-
Catalyst-free four-component domino synthetic approach toward versatile multicyclic spirooxindole pyran scaffolds.RSC Adv. 2019 May 28;9(29):16525-16533. doi: 10.1039/c9ra03214b. eCollection 2019 May 24. RSC Adv. 2019. PMID: 35516360 Free PMC article.
-
Synthesis of highly functionalized thiazolo[3,2-a]pyridine derivatives via a five-component cascade reaction based on nitroketene N,S-acetal.RSC Adv. 2020 Aug 21;10(52):31039-31048. doi: 10.1039/d0ra03910a. eCollection 2020 Aug 21. RSC Adv. 2020. PMID: 35520681 Free PMC article.
-
Efficient regioselective five-component synthesis of novel thiazolo[3,2-a]pyridine carbohydrazides and oxazolo[3,2-a]pyridine carbohydrazides.Mol Divers. 2023 Apr;27(2):667-678. doi: 10.1007/s11030-022-10446-0. Epub 2022 May 19. Mol Divers. 2023. PMID: 35587848
-
C3-Spirooxindoles: Divergent chemical synthesis and bioactivities (2018-2023).Bioorg Chem. 2024 Feb;143:107091. doi: 10.1016/j.bioorg.2023.107091. Epub 2024 Jan 4. Bioorg Chem. 2024. PMID: 38183683 Review.
-
5-Amino-pyrazoles: potent reagents in organic and medicinal synthesis.Mol Divers. 2019 Aug;23(3):751-807. doi: 10.1007/s11030-018-9902-8. Epub 2018 Dec 14. Mol Divers. 2019. PMID: 30552550 Review.
Cited by
-
Silica/APTPOSS anchored on MnFe2O4 as an efficient nanomagnetic composite for the preparation of spiro-pyrano [2, 3-c] chromene derivatives.BMC Chem. 2024 Aug 24;18(1):155. doi: 10.1186/s13065-024-01270-8. BMC Chem. 2024. PMID: 39182154 Free PMC article.
-
Green and Regioselective Approach for the Synthesis of 3-Substituted Indole Based 1,2-Dihydropyridine and Azaxanthone Derivatives as a Potential Lead for SARS-CoV-2 and Delta Plus Mutant Virus: DFT and Docking Studies.ACS Omega. 2022 Nov 18;7(48):43856-43876. doi: 10.1021/acsomega.2c04990. eCollection 2022 Dec 6. ACS Omega. 2022. PMID: 36506171 Free PMC article.
-
Synthetic strategies of heterocycle-integrated pyridopyrimidine scaffolds supported by nano-catalysts.RSC Adv. 2023 Apr 14;13(17):11600-11634. doi: 10.1039/d3ra00922j. eCollection 2023 Apr 11. RSC Adv. 2023. PMID: 37063723 Free PMC article. Review.
References
-
- Rainoldi G., Begnini F., De Munnik M., Lo Presti L., Vande Velde C.M., Orru R.V., Silvani A. Sequential multicomponent strategy for the diastereoselective synthesis of densely functionalized spirooxindole-fused thiazolidines. ACS Comb. Sci. 2018;20:98–105. - PubMed
-
- Velazquez-Campoy A., Todd M.J., Freire E. HIV-1 protease inhibitors: enthalpic versus entropic optimization of the binding affinity. Biochem. 2000;39:2201–2207. - PubMed
-
- Chen H., Shi D. Efficient one-pot synthesis of novel spirooxindole derivatives via three-component reaction in aqueous medium. J. Comb. Chem. 2010;12:571–576. - PubMed
-
- Joshi R., Kumawat A., Singh S., Roy T.K., Pardasani R.T. Synthesis of spirooxindoles through cyclocondensation of isatin and cyclic 1,3-Diones. J. Heterocycl. Chem. 2018;55:1783–1790.
LinkOut - more resources
Full Text Sources

