DBU-Catalyzed One-Pot Synthesis of Nearly Any Metal Salt of Fatty Acid (M-FA): A Library of Metal Precursors to Semiconductor Nanocrystal Synthesis
- PMID: 32258902
- PMCID: PMC7114616
- DOI: 10.1021/acsomega.9b04448
DBU-Catalyzed One-Pot Synthesis of Nearly Any Metal Salt of Fatty Acid (M-FA): A Library of Metal Precursors to Semiconductor Nanocrystal Synthesis
Abstract
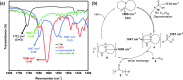
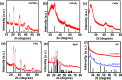
The metal salts of fatty acid (M-FA) are the most widely used metal precursors to colloidal semiconductor nanocrystals (NCs). They play a key role in controlling the composition, shape, and size of semiconductor NCs, and their purity is essential for attaining impeccable batch-to-batch reproducibility in the optical and electrical properties of the NCs. Herein, we report a novel, one-pot synthesis of a library of highly pure M-FAs at near-quantitative yields (up to 91%) using 1,8-diazabicyclo[5.4.0]undec-7-ene or the related nonionic/noncoordinating base as an inexpensive and ecofriendly catalyst in a green solvent medium. The method is highly general and scalable with vast academic and industrial potential. As a practical application, we also demonstrate the use of these high-quality M-FAs in the synthesis of the spectrum of colloidal semiconductor NCs (III-V, II-VI, IV-VI, I-VI, I-III-VI, and perovskite) having absorption/emission in visible to the near-infrared region.
Copyright © 2020 American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures





References
-
- Rossetti R.; Nakahara S.; Brus L. E. Quantum Size Effects in the Redox Potentials, Resonance Raman Spectra, and Electronic Spectra of CdS Crystallites in Aqueous Solution. J. Chem. Phys. 1983, 79, 1086–1088. 10.1063/1.445834. - DOI
-
- Brust M.; Walker M.; Bethell D.; Schiffrin D. J.; Whyman R. Synthesis of Thiol-Derivatised Gold Nanoparticles in a Two-Phase Liquid-Liquid System. J. Chem. Soc., Chem. Commun. 1994, 801–802. 10.1039/c39940000801. - DOI
-
- Murray C. B.; Norris D. J.; Bawendi M. G. Synthesis and Characterization of Nearly Monodisperse CdE (E = S, Se, Te) Semiconductor Nanocrystallites. J. Am. Chem. Soc. 1993, 115, 8706–8715. 10.1021/ja00072a025. - DOI
-
- Battaglia D.; Peng X. Formation of High Quality InP and InAs Nanocrystals in a Noncoordinating Solvent. Nano Lett. 2002, 2, 1027–1030. 10.1021/nl025687v. - DOI
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

