Amphiphilic Cationic Triscyclometalated Iridium(III) Complex-Peptide Hybrids Induce Paraptosis-like Cell Death of Cancer Cells via an Intracellular Ca2+-Dependent Pathway
- PMID: 32258934
- PMCID: PMC7114882
- DOI: 10.1021/acsomega.0c00337
Amphiphilic Cationic Triscyclometalated Iridium(III) Complex-Peptide Hybrids Induce Paraptosis-like Cell Death of Cancer Cells via an Intracellular Ca2+-Dependent Pathway
Abstract
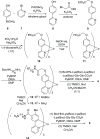
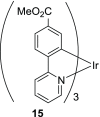
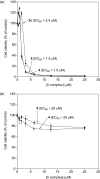
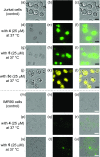
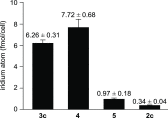
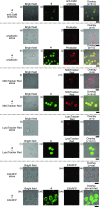
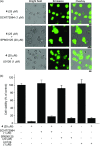
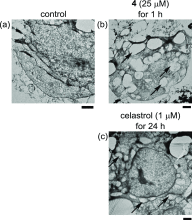
We report on the design and synthesis of a green-emitting iridium complex-peptide hybrid (IPH) 4, which has an electron-donating hydroxyacetic acid (glycolic acid) moiety between the Ir core and the peptide part. It was found that 4 is selectively cytotoxic against cancer cells, and the dead cells showed a green emission. Mechanistic studies of cell death indicate that 4 induces a paraptosis-like cell death through the increase in mitochondrial Ca2+ concentrations via direct Ca2+ transfer from ER to mitochondria, the loss of mitochondrial membrane potential (ΔΨm), and the vacuolization of cytoplasm and intracellular organelle. Although typical paraptosis and/or autophagy markers were upregulated by 4 through the mitogen-activated protein kinase (MAPK) signaling pathway, as confirmed by Western blot analysis, autophagy is not the main pathway in 4-induced cell death. The degradation of actin, which consists of a cytoskeleton, is also induced by high concentrations of Ca2+, as evidenced by costaining experiments using a specific probe. These results will be presented and discussed.
Copyright © 2020 American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures






References
-
- Dedeian K.; Djurovich P. I.; Garces F. O.; Carlson G.; Watts R. J. A new synthetic route to the preparation of a series of strong photoreducing agents: fac-tris-ortho-metalated complexes of iridium(III) with substituted 2-phenylpyridines. Inorg. Chem. 1991, 30, 1685–1687. 10.1021/ic00008a003. - DOI
-
- Flamigni L.; Barbieri A.; Sabatini C.; Ventura B.; Barigelletti F. Photochemistry and photophysics of coordination compounds: iridium. Top. Curr. Chem. 2007, 281, 143–203. 10.1007/128_2007_131. - DOI
-
- Omae I. Application of five-membered ring products of cyclometalation reactions as sensing materials in sensing devices. J. Organomet. Chem. 2016, 823, 50–75. 10.1016/j.jorganchem.2016.09.008. - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

